Metaphase Chromosome
The looped domains coil further
Gene Expression
Prokaryotic Cells
Operons
Lac Opern
Operon ON
Lactose present, Repressor inactive
When there is no Glucose and Lactose is present, then the Operon is ON, because an Inducible Operson (as expression of Lac Z, Lac Y, Lac A genes) is induced by the presence of Lactose
Lactose present, glucose (cAMP level is high), causing an abundant lac mRNA to be synthesized
When lactose is present, the Lac repressor protein binds Lactose, such that it can no longer bind to the operator sequence. RNAP can now bind promoter for transcription of lac operon genes.
The activator protein is present (because an activator protein is required for function) and it's called CAP (Catabolite activator protein). It is activated by cAMP. Adenylyl cyclase forms cAMP from ATP. cAMP binds CAP and CAP helps RNAP to bind promoter to facilitate transcription, so the operon is ON
Operon OFF
Lactose present, Glucose present (cAMP level is low): little lac mRNA is synthesized
If there is no Lactose, then Lac Repressor will be bound to the Operator preventing expression of Lac Operon genes.
The presence of Glucose blocks Adenylyl Cyclase preventing the production of CAMP (if there is no cAMP present then the CAP is inactive, so it can't help the RNAP bind to the promoter.
Lactose absent, Repressor Active
When there is no Lactose, the Operon is OFF, because you need an enzyme. Due to this Transcription, Lac Z, Lac Y, and Lac A is blocked (thus causing the Operon to be OFF).
It's found in E. Coli and is founded by Francois Job and Jacques Monod in 1962. It is an example of both Positive and Negative regulation.
Regulatory Region
Operator
Promoter
Regulatory Gene
Lac I
Stuctural Genes
Lac A
Lac Z
Lac Y
A cluster of functionally realted genes which are involved in the same pathway in the cell consisting of the coordinated control of a single on-off "switch."
Negative Regulation
When the Repressor protein is bound to the Operator sequence, then the gene expression if OFF
Positive Regulation
The Operon gene expression if ON (expression is at a high level when the activator is bound to the operator)
Cell Specific Transcription
Combinatorial Control of Gene Expression
In the Liver Cell, Activator proteins are present that bind to enhancer sequences that increases the expression of Albumin Gene. ON THE OTHER HAND, in the Lens cell, activator proteins are present that bind enhancer sequences of the Crystallin Gene and not the Albumin Gene. In this case (in the Lens), only Crystalline gene has high levels of expression while Albumin has basal or background expression.
Model for the Action of Enhancers and Transcription Activators
Activator proteins bind to distal control elements grouped as an enhancer in the DNA. This enhancer has three binding sites, each called a distal control Element
A DNA-bending protein brings the bound activators closer to the promoter, where General transcription facotrs, mediator proteins, and RNA Polymerase II are nearby.
The activator bind to certain mediator proteins and general transcription factors, helpingthem form an active transcription initation complex on the promoter
Control Elements in DNA
Distal Control Elements
Enhancers
Groupings of distral control elements which are far from the gene they control
Sequences in DNA that are upstream or downstram of DNA and binds to specific transcription factors (like Activators or Repressors)
Proximal Control Elements
Sequences in DNA that is close to promoter and binds to general transcription facotrs
Transcription Factors
Specific
Binds to distal control elements caleld Enhancers and bring changes in the level of transcription
Repressors
Reduces levels of transcription
Activators
Increases levels of transcription
General
It binds to the promoter and regionsn ear the Promter to bring about basal or background level of Transcription
Regulation at Transcription Initiation
A Eukaryotic promoter (commonly includes a TATA box) about 25 Nucleotides upstream from the transcriptional start point
Several Transcription Factors (one that recognizes the TATA box) binds to the DNA before RNA Polymerase II can bind in the correction position
Additional Transcription Factors bind to the DNA along with RNA Polymerase II, forming the transcription initation complex. RNA Polymerase II then unwinds the DNA double helix, and RNA synthesis begins at the start point on the template strand
Gene makes RNA when needed
Differential Gene Expression
Almost all cells in an organism contains the same genes, because they are all the same BUT have differential expression (dependong on what they are and the difference purposes they want to express)
Gene Regulation
Eukaryotic Cells
Nucleosomes
300 nm-Fiber
The 30-nm fiber that forms looped domains that attach to proteins
30-nm Fiber
Interactions between Nucleosomes cause the thin tiber to coil or fold into this thicker fiber
The packaged DNA (in the nucleous) that consist of DNA winding around a Histone protein
Linker DNA
The DNA connecting each nucleosome
Histone
Small protein that DNA wraps around
H1
It's not part of the Nuclesome, but is involved in forming the next level of packaging
Histone Core
H4
H3
H2B
H2A
Chromosomes
Compacted Chromatid that are made of DNA (genes) and proteins
Chromatid
The fibrous double stranded DNA to which proteins are attached to, once it's compcted it formed Chromosomes
10nm Fiber
DNA winds around histones to form Nucleosomes "beads." They are then strung together like beads on a string by linker DNA
Transcription and RNA Processing
linear flow of information from DNA to protein through the formation of mRNA
a process that forms mRNA from DNA
nucleotide in DNA where transcription starts (first nucleotide or +1) on template strand
To the left of the transcription start site nucleotides are numbered by negative numbers
Nucleotides in DNA to the right are labeled by positive numbers
transcription occurs in the nucleus forming pre mRNA and translation in the cytoplasm
sequence AAUAAA is a signal for ribonuclease to make a cut in the newly formed pre mRNA and release it from DNA. At the 5’ end a modified G nucleotide CAP is added, and at the 3’ end a polyA tail is added by polyA polymerase. The 5’cap will be used for translation and the 3’polyA tail helps with stability of the mRNA.
Pre mRNA contains introns and exons. Introns are sequences that need to be removed before translation.
Before the pre mRNA exits the nucleus to be translated it has to remove introns and join together exons. Different genes contain different number of introns.
There is a complex of RNA and proteins called Spliceosome that binds the junctions of the introns and makes cuts to release the introns from the DNA. The exons are then joined together.
Presence of introns help alternate splicing. Different combinations of exons can be generated through removal of different introns to form different mRNAs and hence different proteins.
RNA polymerase II moves downstream, unwinding the DNA and elongating the RNA
transcript 5' to 3'. In the wake of transcription, the DNA strands re-form a double helix.
A eukaryotic promoter commonly includes a TATA box (a nucleotide sequence containing TATA) about 25 nucleotides upstream from
the transcriptional start point.
Several transcription factors, one recognizing the TATA box, must bind to the DNA before RNA polymerase II can bind in
the correct position and orientation.
Additional transcription factors bind to the DNA along with RNA polymerase II, forming the transcription initiation complex. RNA polymerase II then unwinds the DNA double helix, and RNA synthesis begins at the start point on the template strand.
both transcription and translation occur in the cytoplasm in Prokaryotes, mRNA can be immediately translated
termination
Eventually, the RNA transcript is released, and the polymerase detaches from the DNA.
Translation and protein transport
Termination
Protein transport- all of protein synthesis begins on free ribosomes. The different sequence of amino acids tell proteins what their final location will be and their function.
During translation an SPR will bind to the peptide stand and will momentarily pause the synthesis. The SPR will then also bond itself to a receptor protein found in the membrane of ER.
This SRP will then leave the signal peptide which causes the polypeptide synthesis to resume its natural course. At the same time we have translocation of the protein in the ER membrane.
The protein is then cut by an enzyme found in the receptor. Once this happens the polypeptide is folded to its final conformation.
The protein the goes through further folding in the Golgi, it is shipped here from the ER through a vesicle.
After this the protein is then released into the cell so it cal reach its designated location. Whether it be another organelle like the nucleus or mitochondria, or secreted from the cell.
Once the stop codon is reached a release factor will stand in the A site and this will dissociate the complex stopping translation
A stop codon is either UAG, UAA, or UGA. the dissociating of the complex stopping translation is a GTP driven processes as well.
Elongation
After the initiation process comes the elongation step. In the A site of the large ribosomal subunit a new incoming tRNA base-pairs with the mRNA. Many tRNAs will attempt to bind to the codon in the A site but only the appropriate anticodon will bind to the mRNA.
A peptide bond will then form between the amino acid in the P site and the A site. This bond occurs between the amino acid found in the A site and the carbonyl end of the polypeptide in the P site. This bond removes the polypeptide from the tRNA in the P site and adds it to the amino acid in the A site.
The last step in elongation is called Translocation. In this final step the tRNA shift down a site, meaning that the tRNA in the P is shifted to the E site and is released. The tRNA is shifted to the A site is moved the the E site and at the same time the mRNA iOS moved with its corresponding tRNAs. Meaning that the A site will be open for the next appropriate tRNA to bond.
The translocation step will repeat and repeat until it reaches the stop codon, which then leads to the termination process of translation.
Initiation
At initiation a small ribosomal subunit binds to am mRNA. The small ribosomal unit then scans the mRNA until it finds AUG, which is the start codon.
An initiator tRNA, that consists of an anticodon of UAC will base-pair with AUG. This initiator tRNA carries the amino acid MET, which is methionine.
A large subunit then joins and becomes a translation initiation complex. Translation factors are also used here to bring all the translation components together.
The initiator tRNA is located in the P site of the large ribosomal subunit, while the A site is open to receive the next tRNA with the corresponding amino acid.
An initiator tRNA is made up of a single strand of RNA with about 80 nucleotides. When in three dimension the tRNA is shaped in an upside down L and has the anti codon towards the bottom. On the top side of the upside down L it has a corresponding amino acid
The purpose of the tRNA is to bring the correct amino acid to the mRNA during translation.
elongation
RNA polymerase moves downstream, unwinding the DNA and elongating the RNA
transcript 5' to 3'. In the wake of
transcription, the DNA strands re-form a double helix.
initiation
After RNA polymerase binds to the promoter, the DNA strands unwind, and the
polymerase initiates RNA synthesis
at the start point on the template strand.
double stranded with complementary base pairings
Watson and Crick stated that the specific base pairing suggested a possible copying mechanism for the genetic material where each strand wouls be a parent strand with the information to make another strand
the double helix was predicted by the Messleson and Stahl experiment showing us that the helix is semi conservative meaning when the helix replicates each daughter molecule will have an old strand and a newly made strand
in this experiment they found an intermediate band as well as a high density band confirming the double helix was semiconservative
replication
the two strands of the double helix must be separated at the ORI (origin of replication) to be able to form a daughter strand. the ORI is a sequence of nucleotides in DNA
the enzume Helicase seperates the two strands to form the replication bubble, SSB (single stranded proteins) makes sure the DNA stays seperated while another enzyme called Topoisomerase helps relieve any strain
Primase makes RNA primers complementary to the DNA parent strand. This causes DNA polymerase lll to add nucleotides only to the 3' end
A protein called sliding clamp works with DNA polymerase lll and helps keep it on the parent strand so it does not fall off during replication
along the leading strand the DNA polymerase continuously moves forward towards the replication fork
to form the lagging strand multiple RNA primers are laid down and extended by DNA polymerase lll and making okazaki fragment. the primers are then removed by DNA polymerase l and replaced by DNA nucleotides. an enzyme called ligase seals any gaps by connecting nucleotides with phosphodiester linkages
to connect these nucleotides that are in DNA together we have to use phosphodiester bonds using dehydration/ condensation reactions.
the structure
sugar phosphate backbone
phosohodiester bond
nitrogenous base
base pairs are held together by hydrogen bonds
Chargaffs rule: The amount of Adenine equals the amount of Thymine. and the amount of Guanine equals the amount of Cytosine
Hershey and Chase discovered that protein was not the genetic material, and that it was DNA
Fredrick Griffith discovered that bacteria are capable of transferring genetic information through transformation
Metabolism
Photosynthesis
other processes
alternative metods of carbon fixation
plants open stomata at night and incorporate C O2 into organic acids
Stomata close during the day, and C O2 is released from organic acids and used in the Calvin cycle
if stomata are partly closed, the little CO2 that can enter the leaf is fixed using PEP carboxylase (high affinity for CO2) in Mesophyll cells into a 4 carbon compound
CO2 released into a neighboring sheath cell wherein it is fixed using Rubisco and goes through the Calvin cycle to make sugars
C3 plants photorespiration--Rubisco favors to bind O2 instead of CO2, so if CO2 concentration is low, Rubisco will bind whatever O2 is present, releasing CO2; no ATP formed
calvin cycle
produces sugar from C O2 with the help of the NADPH and ATP
addition of 3 CO2 from the atmosphere to RuBP using enzyme Rubisco
forms a 6 carbon unstable intermediate which splits to form 6 molecules of 3 phosphoglycerate
6 molecules of ATP and 6 molecules of NADPH used to form 6 molecules of G3P
5 molecules of G3P continue on to make more RuBP and 1 molecule of G3P leaves the cycle to form glucose and other sugars
stroma; outside thylakoid
light reactions
convert solar energy into chemical energy
cyclic flow of e-
excess NADPH present
only PSI used--as e- are transferred to Fd, instead of forming NADPH they are recruited to the cytochrome complex and plastocyanin molecules of the ETC
movement of electrons leads to formation of ATP by photophosphorylation
Non-cyclic (linear) flow of e-
Photosystem II
electron transport chain
e- from primary acceptor go down plastoquinone (Pq), cytochrome complex,
Plastocyanin (Pc), ferredoxin (Fd)
chlorophyll molecules of photosystem I
formation of ATP by phosphorylation--energy from ETC used to pump H+ into thylakoid space against concentration gradient, and go back down concentration gradient through ATP synthase
photon of light is absorbed by chlorophyll fouind in light harvesting complexes
causes e- to jump to excited state, and go back down to ground state, releasing energy
released energy absorbed by another molecule, and process repeats
main reaction center pair of chlorophyll a molecules (P680) where e- are grabbed by an electron acceptor molecule
splitting H2O (O2 is released)
Photosystem I
photon of light absorbed by chlorophyll causes e- to be excited, as they go back to the ground state energy is released
main chlorophyll a molecules (P700) where e- are grabbed by a primary electron acceptor
electrons go to Ferridoxin (Fd) then on to NADP+ to form NADPH
thylakoid membrane
6 CO2 + 6 H2O + Light energy ---> C6H12O6 + 6 O2
6 CO2 + 18 ATP + 12 NADPH + 12 H2O --->
C6H12O6 + 18 ADP + 18 Pi + 12 NADP+ 6 O2 + 6 H2O + 12 H+
leaves
stomata-microscopic pores for CO2 to enter and O2 to exit
mesophyll cells
choloroplasts
chlorophyll--light harvesting pigments
When pigments absorb light, an electron is elevated from a ground state to an unstable, excited state
Electrons fall back down to the ground state, releasing photons that cause an afterglow, giving off light and heat
CHO
CH3
Porphyrin ring--light absorbing
“head” of molecule; magnesium
atom at center
long hydrocarbon tail inserted in the thylakoid membrane
once pyruvate us made if O2 is available it enters the mitochondria and is oxidized to form NADH and acetyl coenzyme A
alcohol fermentation
Lactic acid fermentation
pyruvate is reduced to form lactate and recycling back NAD+ no CO2 is produces
pyruvate forms acetaldehyde which is reduced to form ethanol. CO2 is released and in the process of reduction electrons forms NADH are transferred to acetaldehyde recycling NAD+
Acetyl CoA adds its 2 carbon group to oxaloacetate producing citrate
isocitrate us oxidized and NAD+ is reduced
after CO2 is released the resulting 4 carbon molecule is oxidized then made reactive by addition of CaO
enzyme hexokinase is used to add a phosphate from ATP to glucose to form gluccose 6P
then converted into fructose 6P
uses enzyme PFK ro convert Fructose 6phosphate to fructose 1,6 biphosphate
6 carbon sugar splits unto 2 molecules of 3 carbons forming DHAP and G3P
DHAP converts to G3P so at the end we have 2 molecules of G3P and 1 molecule of glucose
G3P id oxidized by transfer of electrons forming NADH a phosphate group is attatched to the oxidized substrate
phosphate group is transferred to ADP and G3P is oxidized to the carboxyl group of an organic acid
enzyme relocates the remaining phosphate group
enolase causes double bond to form in substrate by extracting a water molecule
the phosphate group is transferred from PEP to ADP forming pyruvate
CELLULAR RESPIRATION
substrate level phosphorylation
an enzyme reacts with a substrate that has a phosphate group
formation of a product and transfer of the phosphate group from the substrate to ADP to form ATP
C6H1206 +6O2 -----> 6CO2 +6H20 + ENERGY
6O2 is being reduced (gaining electrons) to become 6CO2
C6H1206 is being oxidized (losing electrons)into 6CO2
These electrons are taken by an electron shuttle using NAD to form NADH+, H+
an electron transport chain is used that was energy is released throughout each step
electrons can be directly transferred to Oxygen but this will cause an explosion due to the release of heat and light energy
aerobic respiration
redox reactions
oxidation
reduction
catabolic process that uses food sources to form CO2 and water with the release on energy
used to make ATP
mitochondria
oxidative phosphorylation STEP 3
glycolysis STEP 1
electrons are extracted from the glucose and added to an electron carrier NAD+ occurring in the cytoplasm outside the mitochondria
pyruvate formed in glycolysis enters the mitochondria and is oxidized. The product of oxidation enters the citric acid cycle generating more electron carriers, NADH, FADH2
NADH and FADH2 carries electrons down the electron transport chain and generates ATP through oxidative phosphorylation
occurs in the mitochondria the location of the ETC is in the inner mitochondrial membrane
there are 4 complexes in the ETC complexes 1, 3, AND 4 are H + pumps so they're job is to pump H+ against they're concentration gradient they get this energy because when electrons are transferred down energy is released
H+ in the intermembrane space go back down there concentration gradient through a membrane transport protein called ATP synthase the energy associated with the H+ gradient is used to add an inorganic phosphate to ADP to form ATP
1 glucose can form 30-32 molecules of ATP
pyruvate oxidation and the citric acid cycle STEP 2
shape determines function
Eukaryote
Vesicle
Cytoplasm
Cytoplasmic Streaming
Cytosol
Cell Junctions
Gap Junctions
Desmosomes
Tight Junctions
Golgi Apparatus
Vacuole
Contractile Vacuoles
Central Vacuole
Plant Cell
Plasmodesmata
Chloroplast
Cell Wall
Middle Lamella
Primary Cell Wall
Secondary Cell Wall
Animal Cell
Lysosomes
Autophagy
Phagocytosis
Cilia
Dyenin
Extracellular Matrix
Integrin
Proteoglycan
Fibronectin
Collagen Fibers
Food Vacuole
Mitochondrion
ATP
Peroxisome
Microvilli
Cytoskeleton
Microtubles
Tubulin Dimer
Intermediate Filaments
Keratin
Microfilaments
Actin
Centrosome
Centrioles
Flagella
Plasma Membrane
Ribosome
used in lplants
amylopectin - contains branching
Amylose
beta isomer the OH group is on top of the structure
four fused rings
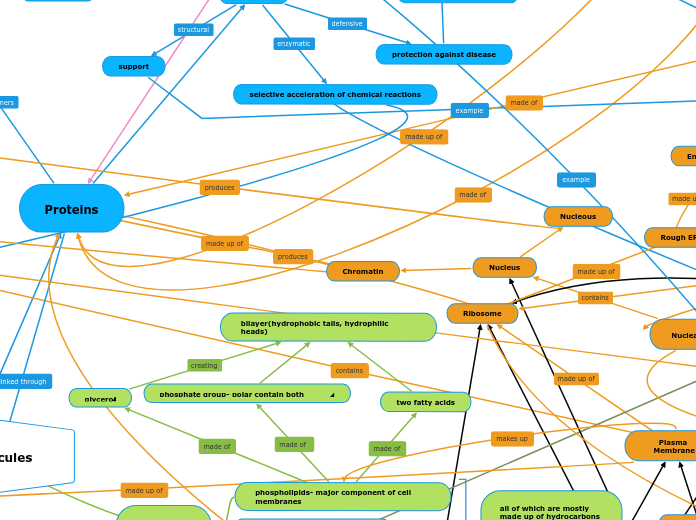
phosphate group- polar contain both hydrophobic and hydrophilic parts
two fatty acids
glycerol
bilayer(hydrophobic tails, hydrophilic heads)
dehydration or condensation synthesis
a disaccharide is formed creating a covalent bond called a glyosidic bond/ linkage
Structure Polysaccharides
chitin
cellulose
made of beta glucose and connected by glyosidic linkages
help together by hydrogen bonds and form microfibrils
storage polysaccharides
dextran
startch
glycogen
connected through 1-4 glyosidic linkages and alpha glucose monomers
used in animals
breakdown using hydrolysis
three fatty acids
made of glycerol
fatty acids
unsaturated- commonly found in plant sources and liquid at room temperature
double covalent bonds are present
isomers
trans isomers having hydrogen on opposite sides of the double bond
cis isomers having hydrogen atoms on the same side of the double bond
saturated- commonly found in animal sources and solid at room temperature
saturated with hydrogen atoms at every position
no double covalent bonds
ester linkage made
all of which are mostly made up of hydrocarbons containg non polar covelant bonds and are generally hydrophobic
Cell signaling
Response
The protein a synthesize/proteins synthesis the mRNA is translated into a specific protein which changes the shape and function of the receiving cell
Transduction
This process used different proteins that are activated throughout the process
The molecule and receptor bind enters the nucleus and binds to specific gene that controls water and sodium flow
The DNA is converted to RNA because of a gene was turned on
Reception
Small and non polar molecules passes through the plasma membrane through a process called simple diffusion
Signal molecule bonds to an internal receptor in the cytoplasm
Intracellular receptors
Signals that are polar or can not diffuse through the membrane must use this kind of receptors to enter the cell and fulfill its purpose
Membrane receptors
Ion channel receptor
An ion channels receptor will remain close until a signaling molecule binds to it. Once that molecule binds the channel will open.
Once this channel is open specific ions are free to flow into the cell and are able to change the concentration of the cell affecting its function and activity
Once the signaling molecule removes itself from the ion channel, the ion channel closes and ions are no longer free to flow into the cell
Tyrosine kinase receptor
These receptors are made of two polypeptides, each polypeptide has the ability to function as a kinase. As a phosphate groups are added to tyrosines this is referred to as a tyrosine kinase receptor the activated receptor cannot interact with other proteins to bring about a response from the cell
These receptors are used when the molecule is hydrophilic and cannot cross the membrane due to the charge and polarity.
G protein linked receptor
Used by non polar signals since they can easily diffuse through the membrane. This receptor is found inside the cell.
GPCR will receive a signal and bind/touch a G proteins to remove GDP to activate GTP after activated the G proteins will change its shape and detach from the GPCR and move to its next task
The GPCR will move along the membrane and attach self to an enzyme. Doing so the GPCR will alter the enzyme shape and function the enzyme will then be activated and complete the steps to cellular response.
In this process GTP will use energy to activate the receptor in which able to change back to GDP and will move back to original position. The process can then be repeated from the beginning.
Lind distance signaling
When the signaling cell is far away from the target cell and must use other forms like the blood stream to send the signaling molecule.
Local signaling
When the releasing cell is in close proximity to the target cell and is able to send the signal molecules through a synapse or extra cellular fluid.
Plasmodesmata
Diffusion of molecules between plant cells
Gap junction
Diffusion of molecules between animal cells
Substrate are held in active site by weak interactions
The active site lowers EA and speeds up the reaction
Substrate are converted to products
Products are released
Active site is available for two new substrate
Enzymes
Enzyme Inhibitors
Feedback Inhibitation
Initial substrate binds to the active site of an enzyme and then the end product goes back and acts as the inhibitor to that same enzyme
Allosteric Inhibitor
Inhibitor that enters the additional binding site of the Allosteric enzyme and stablizes the inactive form.
Cooperativity
Binding of one substrate molecule to the active site of one subunit locks all subunits in active conformation.
Noncompetitive inhibitors
Binds to the enzyme away from the active site and alters the shape of the enzyme, so that when the substrate binds to the active site, it won't be as effective.
Competitive Inhibitors
Mimics the shape of the substrate and competes with the enzyme for the active site. It then blocks the enzyme from sitting on the active site
Optimal pH
Optimal Temperature
Lowered Activation Energy
Catalytic Cycle
Substrate enter the active site; enzyme changes shape
Potential Energy
Food
stores energy
Chemical energy
Molecular Structure
due to position, location, or arrangment
Energy
Thermodynamic
Gibb's Free Energy
Free-Energy Change (∆G)
Equilibrium
No net change occurs
∆G = 0
Endergonic
Energy for Cellular Work
Glutamic Acid conversion to Glutamine
∆G for ATP hydrolysis
Energy required, nonspontaneous
∆G > 0
Exergonic
Energy from catabolism
ATP Cycle
Energy released, spontaneous
∆G < 0
∆G = G(final state) - G(initial state)
G = DH - TDS
H = G + TS
S = Entropy
T = Temperature in Kelvin
G = Gibbs
H = Total Energy (Enthalpy)
Laws of Thermodynamic
Second Law
Every energy transfer or transformation increases the entropy of the universe
First Law
Energy cannot be transferred and transformed, but it cannot be created or destroyed
Surrounding
matter in the rest of the universe
System
Open System
Closed System
matter within define region of space
Kinetic Energy
Energy associated with motion of molecules or objects
Movement of Photon
Light Energy
Molecular Motion
Thermal Energy
There is a consume of energy to build larger, complicated molecules from simpler ones
Polymerization
Subtopic
Anabolic Pathways
Catabolic Pathways
There is a release energy by breaking down complex molecules into simpler compounds
Nuclear Envelope
Nucleus
Chromatin
Nucleous
Metabolic Pathways
Endoplasmic Reticulum (ER)
Smooth ER
Rough ER
About half the sugar made consumed as fuel for cellular respiration. Sugar is transported to nonphotosynthetic cells as sucrose. Excess sugar is stored as starch in chloroplasts or in the cells of roots, tubers, seeds, and fruits
cycle repeated again to to form one molecule of glucose (6 carbons)
Biological Molecules
Main topic
Prokaryote
Archaea
Capsule
Branching in membrane hydrocarbon tails of phospholipids.
Extremophiles
Extreme thermophiles
Thrive in very hot environments
Extreme halophiles
Live in highly saline enviroments
Bacteria
Peptidoglycan in cell wall
Endospore
Inclusion bodies
Gas vacuole
Glycocaly
Nucleoid
Pili
Frimbriae
DNA
Eukarya
Protista
Animalia
Fungi
Plantae
Plants, survive by capturing energy from the sun in photosynthesis
Proteins
amino acids
peptide bonds
polypeptides
amino acid sequence determines protein structure
amino acid will go from buried within protein to the surface
amino acid will go from surface of protein to buried inside
denaturation-protein unfolds back into primary structure (no longer biologically active)
renaturation-reverse conditions and test protein function
only peptide bonds remain
quaternary
2 or more polypeptides come together to form a functional protein
tetramer
hemoglobin
trimer
dimer
intermolecular R group interactions
tertiary
intermolecular R group interactions cause polypeptide to fold
ionic bonds
hydrogen bonds
form intramolecular disulfide bonds through oxidation (only covalent bond between R groups)
hydrophobic/van der waals
secondary
intermolecular hydrogen bond between main chain
beta pleated sheets
alpha helices
primary
intramolecular polar covalent peptide bond through main chain
central (alpha carbon)
side chain (R group)
hydrophobic
nonpolar
hydrophilic
charged
acidic
basic
polar
main chain
amino group
positive charge
zwitterion in neutral pH within cell
hydrogen
carboxyl group
negative charge
functions
movement
coordination of an organism’s
activities
response of cell to chemical
stimuli
support
transport of substances
storage of amino acids
protection against disease
selective acceleration of chemical reactions
Nucleic Acids
polymers made of monomers called nucleotides
nucleoside (do not have a phosphate group
nucleotide (have a phosphate group)
make a phosphodiester bond
one end has a free phosphate group connected to a 5’ carbon of the sugar, while the other end has an OH group connected to the 3’ end of the end sugar. So we call one end of the nucleic acid 5’ end and the other end is the 3’ end.
5 carbon sugar (pentose), a phosphate group, and a nitrogenous base
pyrimidines (C , T) also U in RNA
purines (A, G)
ribonucleic acid (RNA)
Nitrogenous bases used are A, G ,C, U
deoxyribonucleic acid (DNA) : provides direcrions for its own replication
double stranded with complementary base pairing
nitrogenous based used are A, G , C, T
mRNA: messenger RNA which controls protein synthesis
Translation (information from the mRNA is used to make proteins)
Transcription (information in the DNA is used to make mRNA)
Carbohydrates- fuel and building material
include sugars and polymers of sugars
creating glucose in ring formation as well as linear
alpha isomer the OH group on the bottom of the structure
Lipids
steriods
fats- function is energy storage
phospholipids- major component of cell membranes