Unit 2
Understanding Matter
Topic 3.0 Chemical Formulas
What is Mass
What are the physical and chemical properties of matter
Chemical Properties
Examples
Matter
Chemical Properties
Examples: rust
The change of chemical composition in a substance. CAN change appearance AND chemical composition
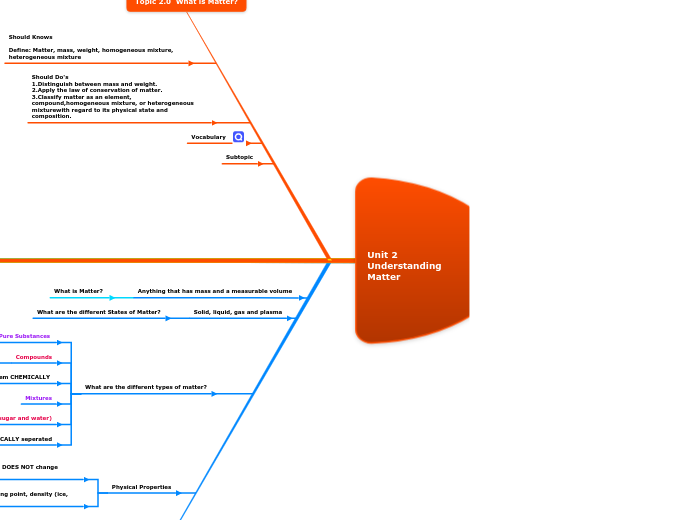
Physical Properties
Examples: melting point, freezing point, density (ice, broken pencil)
CAN change the appearance, It DOES NOT change chemical composition
What are the different types of matter?
They can be PHYSICALLY seperated
Homogeneuous (sugar and water)
Heterogeneous (oil and water)
Mixtures
ONLY can separate them CHEMICALLY
Compounds
Elements
Pure Substances
Solid, liquid, gas and plasma
What are the different States of Matter?
Anything that has mass and a measurable volume
What is Matter?
Category
Cause
Mini Lecture 2.0
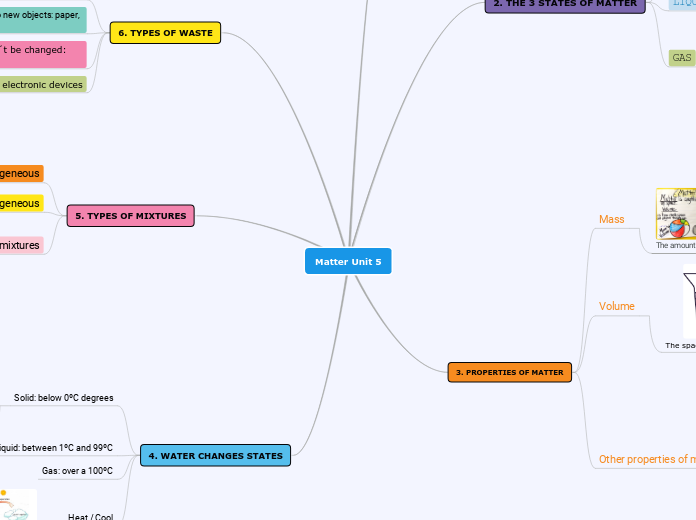
What is Density
Should Do's
Solve Density word Problems
3. Changing in volumes influence density mass remains constant
2. Describe how changing in mass influences density if volume remains constant
1. Manipulate density equation to solve unknown variable
Should Knows
Define Mass, volume and Density
mass = measurement of matter volume
volume = the space matter occupies
density = amount of mass a substance has in a given volume (g/ml )
Topic 2.1 Physical Vs Chemical CHange
Should Do's
1. Describe the difference between physical and chemical changes.
2. Identify metals and nonmetals on the periodic chart.
Should Knows
Define: physical property, chemical
Topic 2.1 Atomic Structure
isotopes are atoms of the same element having different number of neutrons
ions are atoms of the same element having different charges
Both Neutrons and Protons are found in the nucleus
Electrons
Have a NEGATIVE charge
Neurons
Do NOT have a charge
Protons (Atomic Number)
Have a POSITIVE charge
Atoms have two major locations
THE ORBITAL (space around NUCLEUS) - where electrons are
NUCLEUS (center of atom) - Stores protons & neutrons
What is atomic structure?
Topic 2.0 What is Matter?
Subtopic
Vocabulary
Should Do's
1.Distinguish between mass and weight.
2.Apply the law of conservation of matter.
3.Classify matter as an element, compound,homogeneous mixture, or heterogeneous mixturewith regard to its physical state and composition.
Should Knows
Define: Matter, mass, weight, homogeneous mixture, heterogeneous mixture