by Ricardo Diaz 6 years ago
225
BIO311C EVERYTHING
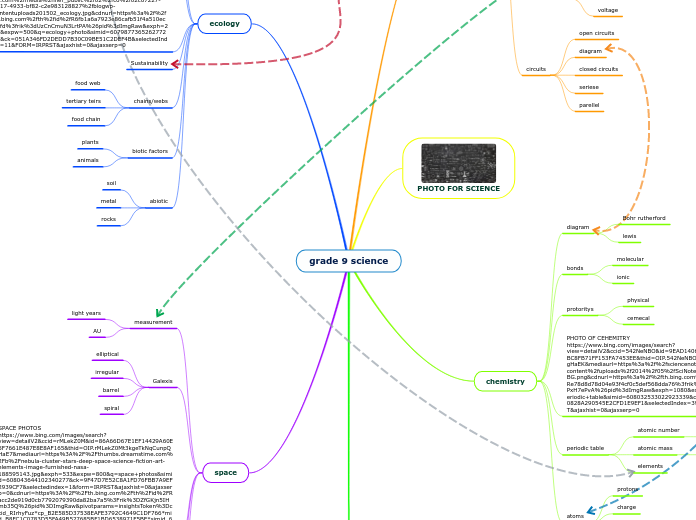
In biological organisms, the most prevalent elements include hydrogen, carbon, nitrogen, oxygen, sodium, magnesium, phosphorus, sulfur, and chlorine. These elements participate in forming various chemical bonds, which can be broadly categorized into ionic, covalent, and metallic.