Test 3 + Extra
Meiosis
Prophase I
The chromosomes condense, and the nuclear envelope breaks down. crossing-over occurs.
Metaphase I
Pairs of homologous chromosomes move to the equator of the cell.
Anaphase I
Homologous chrmosomes move to the oppisite poles of the cell.
Telophase I and Cytokinesis
Chromosomes gather at the poles of the cells. the cytoplasm divides.
Prophase II
A new spidle forms around the chromosomes.
Metaphase II
Chromosomes line up at the equator.
Anaphase II
Centromeres divides. chromatids move to the opposite poles of the cells.
Telophase II and Cytokinesis
A nuclear envelope forms around each set of chromosomes. the cytoplasm divides.
Mitosis
Interphase
(stage 1)
Chromosomes are not visible; chromosomes make a copy of itself. (parent cell).
Prophase
(stage 2)
Copies of chromosomes fasten together;nuclear membrane disappears.
Metaphase
(stage 3)
Chromosomes line up along the center.
Anaphase
(stage 4)
Chromosomes split apart and are pulled to opposite ends of cells.
Telophase
(stage 5)
Chromosomes become hard to see; cells split and 2 nuclear membranes form. (two daughter cells).
Cellular Respiration
- Sugar is broken down to CO2 and H2O, and in the process, ATP is made that can then be used for cellular work
- C6H12O6 + 6O2 --> 6CO2 + 6H2O + ~38 ATP
Photosynthesis
- The process by which green plants and some other organisms use sunlight to synthesize foods from CO2 and H20
- 6CO2 + 6H2O + light energy = C6H12O6 + 6O2.
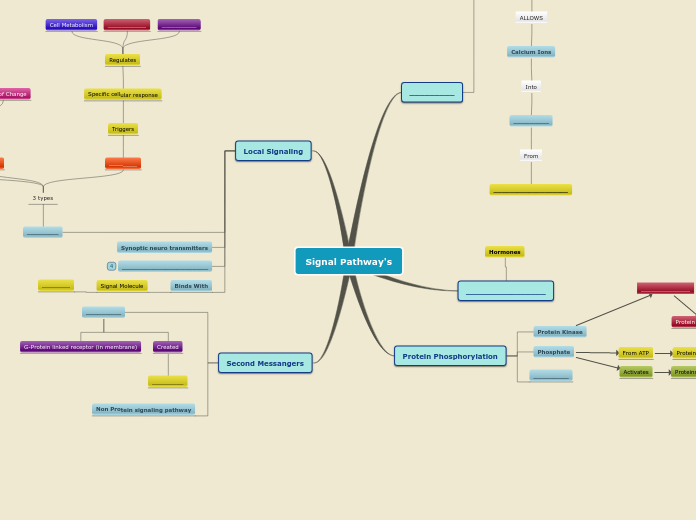
Signal Transduction
Transduction:
- Kinase: Add phosphate group
- Phosphotase: removes phosphate groups
- Adenylyl cyclase: use ATP and makes cAMP = active
- Phosphodiesterase: makes cAMP to AMP = inactive
- Second Messengers (cAMP) will transmitt signals from receptors on cell surface to target cells
Reception:
- G protein inactive with GDP
- GPCR and receptor activates and changes shape. Receptor binds to G protein, removing GDP and attaching GTP ehich activates G-protein
- Activated g-protein leaves receptor and binds to enzyme, changing shape and activating it
- G-protein hydrolyzes off GTP to GDP now Gprotein is inactive and leave enzyme
Long Distance
- Leaves endocrine cell and goes through the blood stream until it reaches target cell that has the receptor
- Hormone signaling
Short distance
Paracrine signaling
- signals leaving secretory vesicles and entering taregt cell
Synaptic signaling
- Neurotransmitters leave synapse and are taken up by target cell
- electrical signal
Action potential
Depolarization:
- Inside less negative
- Sodium Na+ channels open
Hyperpolarization:
- Inside more negative
- Opening of K+ channels
Ions Pumps
H+
- Also called proton gradient
- Concentration difference between the inner mitochondrial membrane and the outer mitochondrial membrane.
Sodium-potassium
- Potassium inside, Sodium outside
- Cell is more negative outside
- For every 3 sodium ions pumped put, 2 potassium ions are pumped in
- Requires energy ATP
- Phosphate groups get attached to protein and changes shape to allow either Na/K in/out
- Sodium in, Post
Endo/Exocytosis
Endocytosis
- going in the cell
Receptor-mediated
- receptor binds to proteins and makes coated vesicle
Pinocytosis:
- liquid gets in vesicle
Phagocytosis:
- food going in with macrophages
Diffusion
Active Transport:
molecules going against concentration gradient. Need help of proteins and ATP
Facilitated Diffusion:
Ions going across a membrane. Need the helps of proteins
Simple/Passive diffusion:
Regular non-polar molecules crossing the membrane
Organelles & Functions
- Nucleus: directs the activity of the cell and stores DNA
- Mitochondria: produces energy through cellular respiration
- Rough ER: transport and storage. Protein synthesis
- Ribosomes: synthesizes proteins (made up of RNA and proteins)
- Smooth ER: creates lipids, enzymes and sex hormones
- Chloroplast: Captures energy from sunlight and converts it into chemical energy to supple cell with food. Also makes glucose
- Golgi Apparatus: responsible for directing molecular traffic in the cell; modifies, sorts and transfers molecules
- Lysosomes: contains enzymes that digest waste and "clean up" the cell
- Plasma Membrane: outer membrane of the cell that lets things in and out of the cell
- Cilia: help to sweep away fluids and particles
- Flagellum: allows cell to move; propels cell (like sperm)
- Peroxisome: single membrane sacs filled with enzymes. form hydrogen peroxide and convert to water
- Vacuole: stores water
- Vesicle: single membrane enclosed sacs
- Cytoskeleton: allows cells to change shape, move, and supports the cell
- Macrophages: have a lot of lysosomes, break things down
- Lymphocytes: make antibodies, made by B cells
- T-cells: communicate and tell each other what to do and make/release proteins, have to have rough ER
Cytoskeleton: held together with weak interactions so they can assemble/dissemble whenever
Intermediate Filaments
- Proteins super coiled into thicker cables
- Maintain cell shape
- Anchorage of nucleus and other organelles
- Formation of nuclear lamin A
Microfilaments
- Made of actin
- Maintain cell shape and changes
- Muscle contraction
- Cytoplasmic streaming
- Cell motility and division
- At base of plasma membrane
- Motor protein: Myosin
Microtubules
- Made of tubulin
- Maintain cell shape
- Cell motility
- Chromosome movement in cell division (centrioles made up of microtubules
- Organelle movement (cilia and flagella motion mediated by microtubules
- Motor protein: kinesin
Lysosomes
- Break down food and covalent bonds and form monomers.
- Hydrolysis reaction
- Nucleases break phosphodiester bonds
- Enzymes only work if the pH is acidic
Structure of smooth ER
- Enzymes present in smooth ER help add hydrogen through hydroxyl group so that the toxins become soluble and leave the body
Nuclear Lamin A
- Protects nuclear membrane
- If mutated, the lamin A falls apart so the membrane falls and the cell dies prematuring
Test 2
Endomembrane system
Endomembrane system
- Translation starts on free ribosome and completed inside the ER
- SRP to ER to golgi
Post-translation:
Can go to
- Nucleus
- Mitochondria
- Peroxisome
- Plastids
Microscopes
Light Microscope
- Magnification approximately 1000
- Resolution 200 nm
Electron Microscopes:
- Better
- Magnification approximately 250,000
- Resolution
Transmission Electron Microscopes (TEM)
- Focus on beam of electrons through a specimen
- Used mainly to study the internal structure of cells
Scanning Electron Microscopes (SEM)
- Focus a beam of electrons onto the surface of a specimen, providing images that look 3D
Gene Expression
Translation
- Occurs in nucleus for pro (coupled with transcription)
- Occurs in cytoplasm for Euk
- mRNA decoded to make amino acids in a polypeptide chain
- tRNA has a sequence of three nucleotides called an anticodon, which can bind to specific mRNA codons
- Amino acid attaches to 5' and tRNA carries amino acid for translation
- mRNA codons each have a specific amino acid to them
- EPA site
- Amino acyl tRNA synthase = adds amino acids to tRNA
- Peptidyl transferase = enzyme that forms peptide bonds with amino acids
Mutations
Frameshift:
insertion & deletion
Nonsense:
introduces stop codon
Missense:
change in amino acid
Silent:
no change in amino acid
Transcription
- Occurs in nucleus for both Pro and Euk
- RNA polymerase in Pro
- RNA polymerase 2 in Euk
- RNAP binds to promoter (transcription factors help in Euk and euk has TATA box)
- Promoter is upstream = negative
- Transcribes DNA and converts into premRNA or mRNA
- RNA is made 5'-3' so it transcribes 3'-5' strand of DNA
RNA Processing for Eukaryotes
- Add 5' cap
- Add 3' poly A tail helps mRNA not get degraded
- cut out introns with spliceosome and leave exons only
- UTR (untranslated region) just there
- Exons leave nucleus and go to cytoplasm for translation
Replication
- In nucleus for Eukaryotes
- In cytoplasm for Prokaryotes
* Starts at the origin of replication
- Helicase: unwinds DNA & breaks hydrogen bonds in nucleotides
- SSB: keeps DNA unwound so it doesn't fold again
- Topoisomerase: relieves strain of DNA from the side
- RNA Primase: drops RNA primers
- DNA Polymerase 3: adds DNA nucelotides in 5'-3' direction and makes okazaki fragments
- DNA Polymerase 1: takes RNA and makes it into DNA
Ligase: fills in any gaps and makes phosphodiester bonds through dehydration/condensation reactions
Eukaroytes
Endosymbiotic Theory:
The organelles in eukaryotes cells evolved through symbiosis of prokaryotes. Examples: Mitochondria and chloroplasts have DNA and double membranes
Eukarya
- Nuclear envelope present
- Has membrane enclosed organelles
- Membrane lipids are unbranched hydrocarbons
- Has several kinds of RNA polymerase
- Has introns
- Has histones associated with DNA
Plants
Membrane: Primary and secondary cell walls, then plasma membrane
Junction
- Plasmodesmata: same as gap in animals
Animal
Membrane: extra cellular matrix
Junctions
- Tight: nothing passes
- Gap: everything can go through
- Desmosome: only some can go through
Prokaroytes
Archaea
Thermophiles = can handle extreme temps
Halophiles = are in extreme salty conditions
Methalogens = release methane
- Membrane lipids have some unbranched carbons
- Have a cell wall
- Several kinds of RNA polymerase
- Has circular chromosomes
Bacteria
- No nuclear envelope
- Has peptidoglycan in cell wall
- Membrane lipids are unbranched hydrocarbons
- Has circular chromosomes
- One kind of RNA polymerase
Experiments
Messelson and Stahl
- First round: DNA semiconservative or dispersive
- Second round: DNA is semiconservative
Hershey and Chase
- Protein: with sulfur
- DNA: with phosphorus
- Phage radiolabeled, bacteria infected, phage grown, centrifuge
- Conclusion: DNA is genetic material
Griffith Experiment
- injected with rough strain = mouse lives
- injected with smooth strain = mouse dies
- injected with heat-killed smooth strain = mouse lives
- injected with rough + heat-killed smooth = mouse dies
Miller-Urey Experiment: was a chemical experiment that simulated the conditions thought at the time to be present on the early Earth, and tested the chemical origin of life under those conditions.
Test 1
EXAM & QUIZZES
* Lowest to Highest = oxygen, water, protein, mitochondria, cell, tissue, organism
* Polysaccaharides, triglycerides, and proteins are all made condensation/dehydration synthesis
* Alpha glucose (OH on bottom) is present in glycogen and starch
* Beta glucose (OH on top) is present in cellulose
* Peptide bonds are in proteins, phosphodiester bonds in DNA
* DNA has deoxyribose sugar and RNA has ribose sugar
* Oxygen has a partial negative charge, carbon has a partial positive charge. Carbon is bonded to each oxygen by polar covalent bonds
* Water has high specific heat because of absorption and release of heat when hydrogen bonds break and reform
* Fats are made of fatty acids and glycerol
* Non-polar amino acids will be in the interior of proteins
* Lactose will dissolve in water through forming hydrogen bonds with water
* Phosphodiester bonds link phosphate to 2 deoxyribose sugars by covalent bonds
* Example of hydrolysis is digesting starch to form glucose
* Glycogen has alpha glycosidic linkages
* Cholesterol and Unsaturated fatty acids
Nucleic Acid
RNA
- A, G (purines)
- U, C (pyrimidines)
- single stranded
- sugar: ribose
- helps w/ translation
DNA
- A, G (purines)
- T, C (pyrimidines)
- double stranded
- sugar: deoxyribose
- stores hereditary info
Nucleotide: nitrogenous base, sugar and phosphate
Nucleoside: only nitrogenous base and sugar
Proteins
Structures
Primary: sequence of amino acids in a polypeptide chain
Secondary: Alpha folded helices or beta pleated sheets. Hydrogen bonds keep them together-stabilize.
Tertiary: Highest level polypeptide can fold to. Interaction between R groups
Quaternary: 2 or more tertiary structures (polypeptide chains)
Monomers: amino acids
Polymers: protiens
All have amino and carboxyl group
Different R groups
Polar: OH, SH, NH2 with hydrogen bonds
Non-polar: H, CH3 with van der waals and hydrophobic interactions
Acidic: negative charge
Basic: positive charge
Lipids
Fatty Acids
- Triglyceride= 3 fatty acids + 1 glycerol (fat & oil)
- Phospholipid = Phosphate group + 2 fatty acids
Steroids
Cholesterol: Ampipathic. Want high High-density-lipoprotein (HDL, good) and low Low-density-lipoprotein (LDL, bad) for cholesterol. Cholesterol make membranes more fluid but too much will make membrane saturated.
Saturated
Butter: Solid, trans fat. Fatty acids packed tightly together. Hydrophobic interactions keep them together. Has trans fat. No double bonds.
Hydrogenated: converts unsaturated fats to saturated fats, some trans fat present. Good to bad.
Unsaturated
Oil: Liquid, cis fat. Has kink because of the double bonds present and has spaces between the fatty acids, not tightly packed. Unstable hydrophobic interactions. Better to consume.
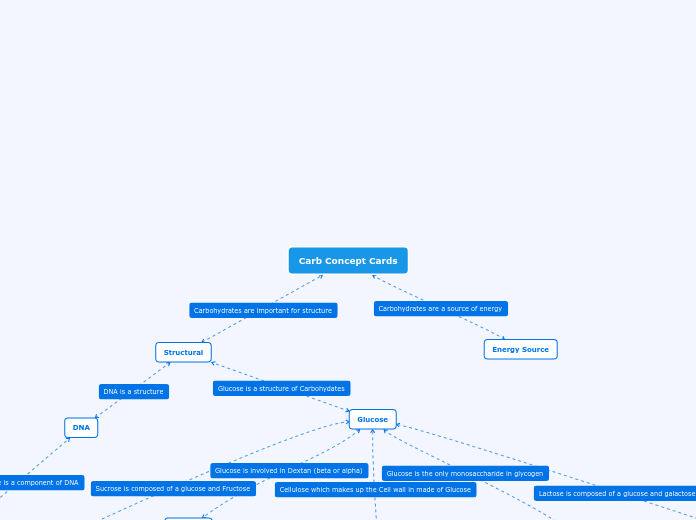
Carbohydrates
Polysaccharides
Storage: glycogen, starch, dextran
Starch: plant storage in the chloroplasts Amylose - linear chains kept together with alpha 1-4 linkages Amylopectin - branched out and has alpha 1-4 and alpha 1-6 linkages
Glycogen: animal storage in the mitochondria It's much more branched than starch, allowing for multiple access points for glucose to come out when body need it
Structure: cellulose, chitin
Chitin: Animal. found in the exoskeleton of arthropods and walls of fungi
- can be used for surgical threads
Cellulose: Plants. straight parallel glucose chains held together by beta 1-4 glycosidic linkages and hydrogen bonds
Disaccharides
- 2 monosaccharides coming together
Sucrose = glucose + fructose together by 1-2 glycosidic linkage
Maltose = glucose + glucose together by 1-4 glycosidic linkage
Monosaccharides
- Glucose, fructose, galactose
Glucose = C6H12O6 (a hexose, has 6-carbon sugars)
OH on top = Beta glucose
OH on bottom = Alpha glucose
In linear glucose, Carbon 2,3,4,5 are asymmetric
Monomer- monosaccharides
Polymer- carbs
covalent bonds hold polymers together
Breakdown of polymers
- Hydrolysis reaction
- Add water, breaking a bond
- use enzymes to break covalent bonds and make individual monomers
- long polymer to short polymer
Synthesis (making) of polymers
- Condensation (dehydration) reaction.
- Removes water molecule, forming a new bond
- short polymer to long polymer
Organic Compounds
Functional groups:
Hydroxyl, OH
Carbonyl, C=O
Carboxyl, COOH
Amino, NH2
Sulfhydryl, SH
Phosphate, OPO2-3
Methyl, CH3
Isomers:
Same formula but different
arrangement of molecules
Enantiomers: mirror images of each other. Assymteric- carbon that has 4 different groups of atoms attached
Geometric: look the same but different spacial orientation. Molecules will be switched
Structural: different structures/bonding arrangement
Carbon Skeletons
- length
- branching
- double bonds
- rings
Water & it's properties
Bond for water : Polar covalent
Hydrogen Bonding:
- Hydrogen bonds break when heat is absorbed
- Hydrogen bonds formed when heat is released
Density: liquid water denser than solid
Heat of vaporization: amount of energy needed to change one gram of a liquid substance to a gas at constant temperature.
High humidity prevents evaporation
High specific heat: amount of heat needed to change one gram of a substance by 1 degrees Celsius
Chemical Bonding
Strongest to Weakest Bonds
- Ionic
- Ion-Dipole
- Hydrogen Bonding
- Dipole-Dipole
- Van der Waals
Ionic
Full charge. Transfer of electrons
Ions of opposite charges form ionic bond.
Negative = anion
Positive = cation
Ionic compound are soluble
Covalent
Non-polar
Similar electronegativity and share electrons equally. No charge
Bonds: C-C, O-O, C-H
Van der Waals: present in all substances
Hydrophobic: interaction of nonpolar substances in the presence of polar substances. Help proteins fold
Polar
Different electronegativity and share electrons unequally. Partial charge
Bonds: O-H, O-C, N-H
Hydrogen Bond: dipole-dipole but stronger
Polar covalent molecules containing H directly attached to O, N or F
Dipole-Dipole
Attraction of partial positive atom and partial negative atom