Chemistry 11 unit 2 - Chemical Reaction
Oxidation and reduction, Reactions or Redox reaction
2HCl(ap)+2Na(s) - 2NaCl(aq)+H2
2Cl- --2Cl- = spectatorion (did nothing)
2H+ + 2e - H2 (o) = reduced
2Na - 2Na (+1) + 2e +oxidized
Na more reactive than hydrogen, so it replace H
Reduction - Gain of electrons from an atom/ion.. / more hydrogen bond
O2 +4e - 2O-2 (reduced
Oxidation - Loss of e- from an atom / more bonds to oxygen
2Mg - 2Mg^+2 = -4e- (oxidized)
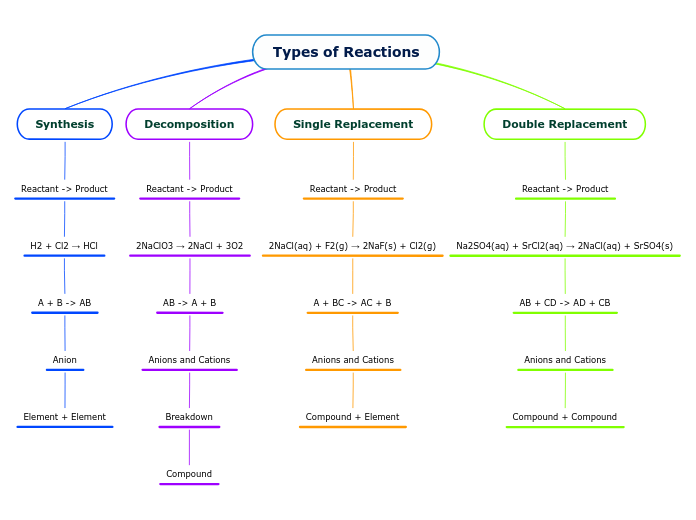
5 Main Types of Chemical Reactions
Combustion: Oxide creation (O2)
Double Displacement: AB+ CD -AC+BD
Single Displacement: AB+CD - AC+BD
Decomposition: AB - A+B
Synthesis: A+B -AB
Balancing Equations
Always balance C, O2, H2, Cl2, Al last
H2So4+2NaOH - NaSo4+2H2O
If a polyatomic exist in the same form on both side, count them as one element
How the same amount of each element on reactant and product side
Reflects the law of conservation of matter
Double Displacement RXN (RXN) = Reaction
A double displacement will occur if the dissociated hydrated ions come out of solution
This can happen in 3 ways
A covalent gas is formed
2) A covalent liquid (mostly H2O) is formed
1) An insoluble solid (precipitate) for mass - refer to solubility table
Small ions with big charge tend to be insoluble because the attraction between is very strong
Note precipitates, do not dissociate so are written associated in total ionic equation
2 aqueous (aq) ionic solution = NR
CaCl2(aq) +KBr (aq) -KCL(aq) + CaBr2 (aq)
Positive go with negative
Then check the solubility table
This case nothing changed, so it's an NR (No Reaction)
Always to write the right equation by using the crossover rule