Chemistry Part 2
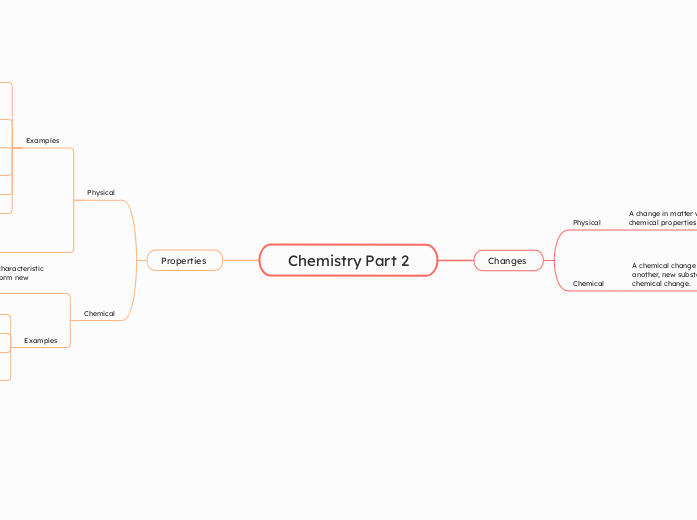
Properties
Heat of combustion
The amount of heat released during the process of oxidation or combustion
Acidity
The level of Acid in a substance
Toxicity
The quality of being toxic or poisonous.
Flamibility
The ease with which a material is ignited
Chemical properties describe the characteristic ability of a substance to react to form new substances
A Physical property is a characteristic of a substance that can be observed or measured without changing the identity of the substance.
Examples
Conductivity
The ability of a substance to conduct electricity.
Taste
What the object Tastes like when consumed.
Luster
The way an substance reflects light. If the object is metallic or not.
Texture
The feel, appearance, or consistency of a surface or substance.
Density
How consistent a substance is measured by mass times volume.
Color
The color of an substance
Changes
Chemical
A chemical change is a change of substances into another, new substances are created with a chemical change.
Digesting food
Rusting of Iron
Baking cookies
Physical
A change in matter which does not alter the chemical properties of the substance.
Examples
Cleaning your self
Mixing drink mix into water.
Cutting up paper