by Tina Bratic 25 days ago
136
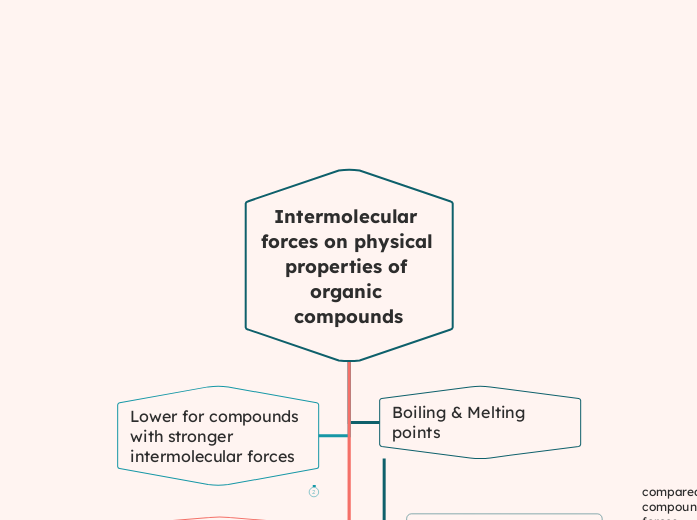
Intermolecular forces on physical properties of organic compounds
Intermolecular forces significantly influence the physical properties of organic compounds. Molecules with stronger intermolecular forces tend to be solids or liquids at room temperature, while those with weaker forces are often gases.