by Farah Najwa 4 years ago
239
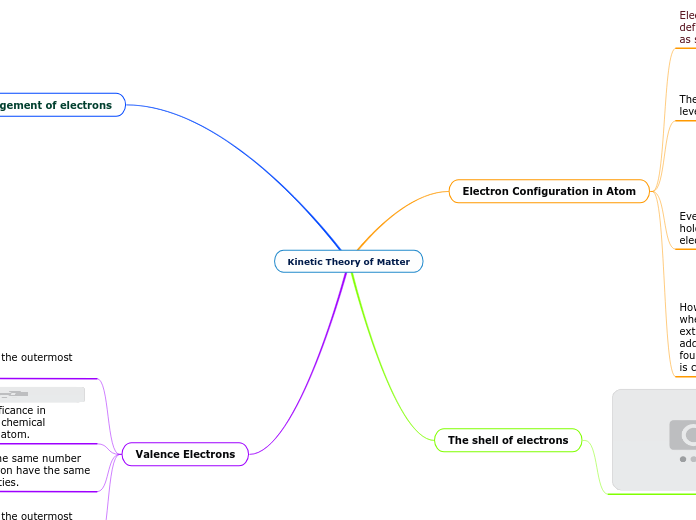
Kinetic Theory of Matter
The arrangement and behavior of electrons in an atom play a crucial role in determining its chemical properties. Electrons are organized into shells, each capable of holding a specific number of electrons, with the first shell holding up to two, the second up to eight, and the third up to eighteen.