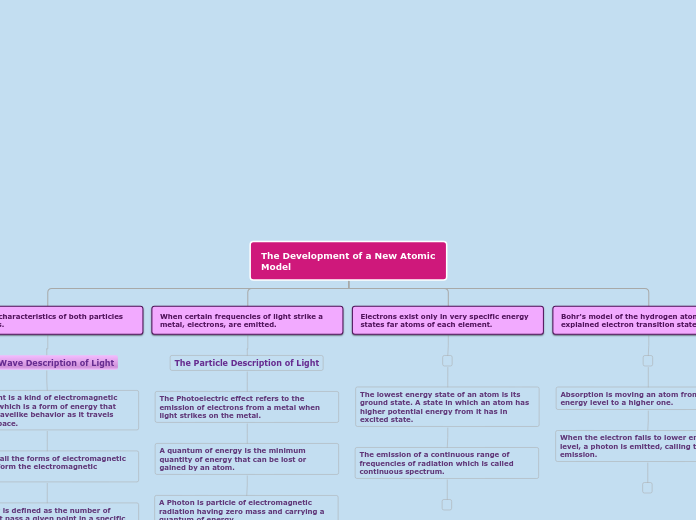
The Development of a New Atomic Model
Bohr's model of the hydrogen atom explained electron transition states.
Electrons exist only in very specific energy states far atoms of each element.
When certain frequencies of light strike a metal, electrons, are emitted.
The Particle Description of Light
The Photoelectric effect refers to the emission of electrons from a metal when light strikes on the metal.
A quantum of energy is the minimum quantity of energy that can be lost or gained by an atom.
A Photon is particle of electromagnetic radiation having zero mass and carrying a quantum of energy.
Light has characteristics of both particles and waves.
The Wave Description of Light
Visible light is a kind of electromagnetic radiation which is a form of energy that exhibits wavelike behavior as it travels through space.
Together, all the forms of electromagnetic radiation form the electromagnetic spectrum.
Frequency is defined as the number of waves that pass a given point in a specific time usually the second.
Wave length is the distance corresponding points on adjacent waves.