Floating topic
Combined Gas Law:
(P1V1)/T1 = (P2V2)/T2
SCH3U0
Solutions & Solubility
Stoich and Solutions
Expanding to include concentrations
and volume into regular stoichiometry problems
Formulas:
n = m/M
c1v1 = c2v2
C = n/v
Chemical Equations
3) Net Ionic Equation
'Spectator Ions' removed
Spectator Ions are ions that don't
appear on both sides of the equation
2) Ionic Equation
Each Ion separated
1) Balanced chemical equation
Creating Solutions
Two liquids mix, one solid (precipitate)
Dilutions
Dilution Formula:
C1V1 = C2V2
Diluting a stock solution of HCL(aq)
- Add 250mL or more of distilled water
up to the fill line
- Measure and add 250 mL of HCL (aq)
to volumetric flask
- Add approx. 500 mL of distilled water
to volumetric flask
- Obtain 1000mL volumetric flask
From a solid
Creating 500 mL of a
mol/L of NaOH
- Obtain 500mL volumetric flask
- Add 250 mL or more to reach the fill line
- Cap and invert to mix
- add approx. 250 mL of
distilled water to flask
- Place measured amount of solid in flask
Concentration
How much of solute in a solution
in terms of moles per litre(mol/L) or "M".
Molar Concentration Formula
Concentration = # of moles/volume
or (c = n/v)
Percentage Concentrations
Other Concentrations
ppm (parts per million) =
- 1 g/m3
- 1 g/1000L
- 1 mg/L
- 1 mg/kg
Used for various purposes in industries
that use different standards
3 types
Mass/Volume % (m/v)
Volume/Volume % (v/v)
Mass/Mass % (m/m)
Components of a solution
Solvent
Medium in which solute is being dissolved
(water, alcohol, etc.)
Solute
Substance being dissolved into a solvent
(sugar, salt, etc.)
Characteristics of solutions
Particles cannot be clearly seen
Cannot be filtered
Unsaturated Solution -
Solute less than fully saturated
Saturated Solution -
No more solute can be dissolved
at the given temperature
Polar molecules dissolve in polar
non-polar dissolve in non-polar
May be slightly coloured
Transparent (Clear if aqueous)
Homogenous Mixture
- Can be separated into components
- Looks the same throughout
Gases & Atmospheric Chemistry
Dalton's Law of Partial Pressure
The pressure that each gas
in a mixture exerts is it's partial pressure
Partial Pressure is used to
determine the concentration
or fraction in a mixture
P(total) = P1 + P2 + P3...
Gas Stoich
Expanding to include pressure and temperature into regular stoichiometry problems.
Formulas needed:
The "Ideal" Gas Law
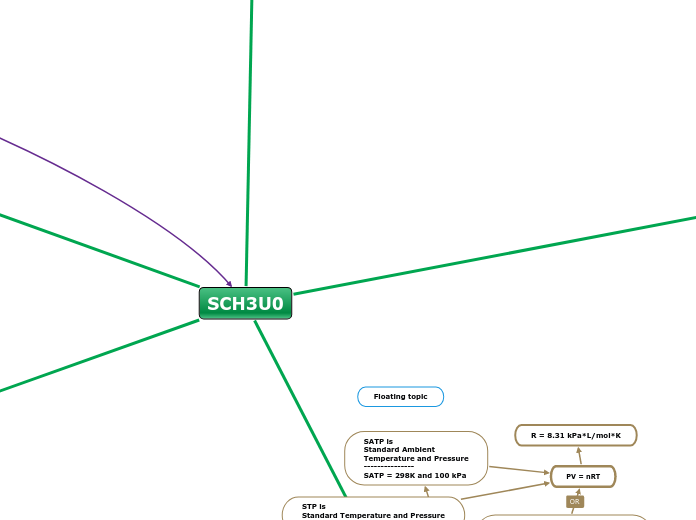
SATP is
Standard Ambient
Temperature and Pressure
---------------
SATP = 298K and 100 kPa
STP is
Standard Temperature and Pressure
STP = 273K and 101.3 kPa
Pressure multiplied by volume
is equal to the number of moles
times the universal gas constant
and temperature
PV = nRT
R = 8.31 kPa*L/mol*K
Gay-Lussac Law
The pressure of a gas varies
directly with the temperature
of the gas.
(At a constant volume)
P1/T1 = P2/T2
Charles' Law
Temperature
Absolute Zero:
Theoretical Temperature where
all matter would freeze.
Absolute Zero is "-273 C" or "0 K"
Temperature recorded in 3 ways:
Celsius (C)
Fahrenheit (F)
Kelvin (K)
K = C + 273
The more kinetic energy the particles
have, the higher the temperature
The volume of a fixed amount
of gas varies directly with
the temperature of the gas
(constant pressure)
V1/T1 = V2/T2
Calculations always done in Kelvin
Boyle's Law
The volume of a gas varies
inversely with with pressure
(at a constant temp)
Shampoo bottle exploded
on plane -
Altitude increases, pressure decreases,
making the volume increase
P1V1 = P2V2
Kinetic Molecular Theory
Pressure = force/area
Can be measured in:
Kilopascals (kPa)
Pascals (Pa)
Atmospheres (atm)
Millimeters of Mercury (mmHg)
Torrs
Gas particles are in constant
motion and have perfectly elastic
collisions
(in closed systems)
Multiple collisions increase pressure
Background/Intro
Gasses are the only
compressible state
States of substances depend
on the forces between the particles
If the forces are strong - solid
If the forces are weak - Liquid or gas
Chemical Trends & Bonding
Bonding
Polar Covalent Bond
For a molecule to be polar,
it must be:
- Containing a polar bond
- Molecule does not have symmetry
An uneven distribution of charges
due to unequal sharing of electrons
Intermolecular
Ionic Bonds
Intramolecular
Covalent Bonds
1. Hydrogen bonding
2. Dipole-dipole
3. London forces
Bonding Capacity
Groups (4-8) = 8 - # of valence electrons
Groups (1-3) = # of group
related to # of valence electrons
Lewis-Dot Bonding/Structures
Periodic Trends
PERIODIC LAW:
When elements are arranged by increasing atomic number, they fall into categories with similar chemical/physical properties
Atomic Radius
Increases 🡳 and 🡰
Factors affecting size
Strength of attraction
of valence electrons
Shielding effect -
multiple energy levels
# of shells/energy levels
Half of distance from nucleus to valence
Stoichiometry
Stoich Problems
Molar Ratio:
Moles of unknown/moles of known
Percent Yield
Tells us how 'efficient' a reaction was
Actual Yield:
Actual amount
of product formed
Theoretical Yield:
Calculated or 'expected' amount
of product formed
% Yield = Actual/Theoretical
Limiting/Excess Reagent
To determine which reactant
will be consumed first
Divide moles of each reactant
by coefficient of reactant to find # of "cycles"
Reactant with more cycles is excess
reactant with less is limiting
Given both reactants' masses
Find mass of either reactant(s) or products
Use formulas:
(n = m/M) and (n = N/Na)
Empirical & Molecular Formula
Molecular
Actual ratio for formula
of a compound
Empirical or 'Simplest'
Formula for a compound in the
smallest whole number ratio
Percent Composition (%)
% composition =
(Mass of individual element/total mass) x 100
Moles
Formulas
# of moles = # of entities/Avogadro's constant
or n = N/Na
# of moles = mass(g)/Molar mass(g/mol)
or n=m/M
1 Mole (or "mol) is
~6.02 x 10^23
**Avogadro's Constant**
*The unit used to measure
amounts of any substance.
Chemical Reactions
Nomenclature
Polyatomics and diatomic molecules
Balancing Chemical Equations
Set of rules for
naming compounds
Types of Reactions
Combustion
- Burning of hydrocarbons
- Complete combustion produces CO2 and H2O
Double Displacement
Neutralization Reactions
Base + Acid 🡲 Salt + Water
Precipitation Reaction
Always has a solid and
an aqueous product
Single Displacement
- Element reacts with a compound
and displaces the 2nd element to
form a new compound and an element
Decomposition
- Reactant broken down into
2 or more products
Synthesis
- 2 different molecules/atoms
combine to produce a compound