door Liang Koo Vilca 3 jaren geleden
145
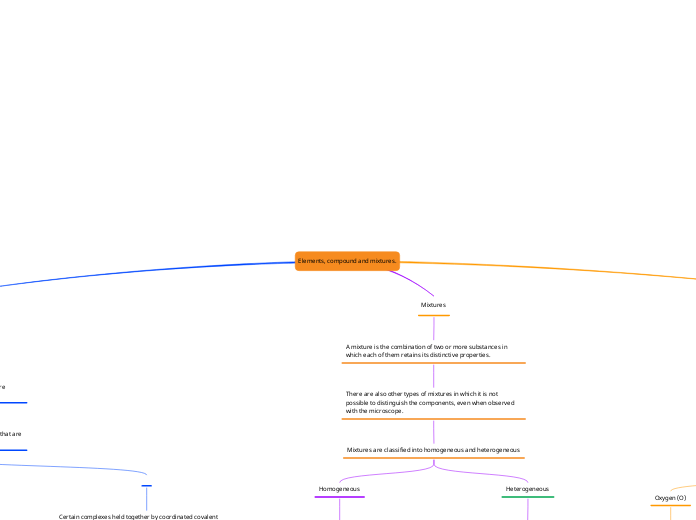
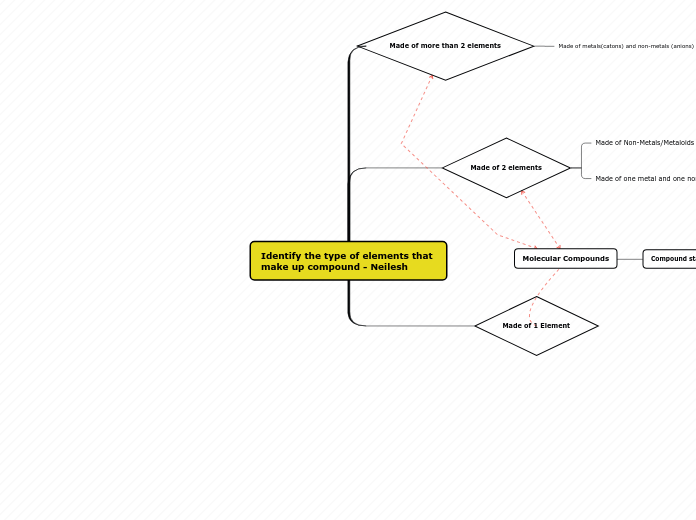
Elements, compound and mixtures.
Mixtures are combinations of two or more substances where each retains its unique properties. These can be homogeneous, where the mixture is uniform and its components are indistinguishable to the naked eye, or heterogeneous, where the components remain identifiable.