door Ana Lucía Cedeño Tejeida 3 jaren geleden
255
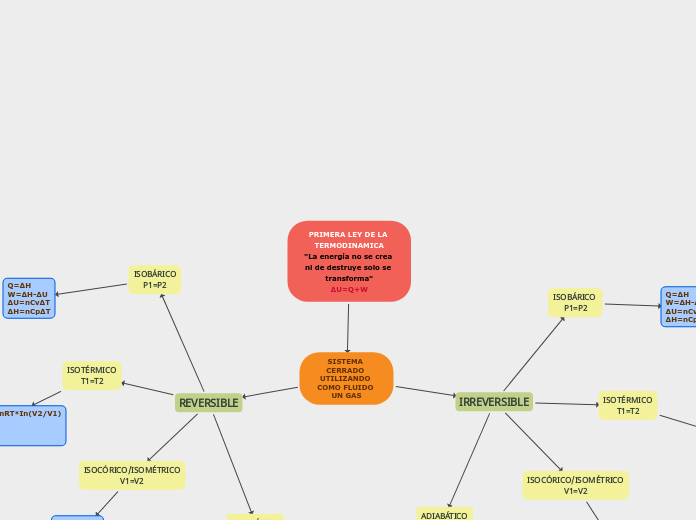
PRIMERA LEY DE LA TERMODINAMICA "La energía no se crea ni de destruye solo se transforma" ΔU=Q+W
Thermodynamics is a fundamental branch of physics that deals with the principles governing the transformation of energy in various systems. The first law of thermodynamics states that energy cannot be created or destroyed but can only be transformed from one form to another.