Unit 1
Transition Mechanisms
Main topic
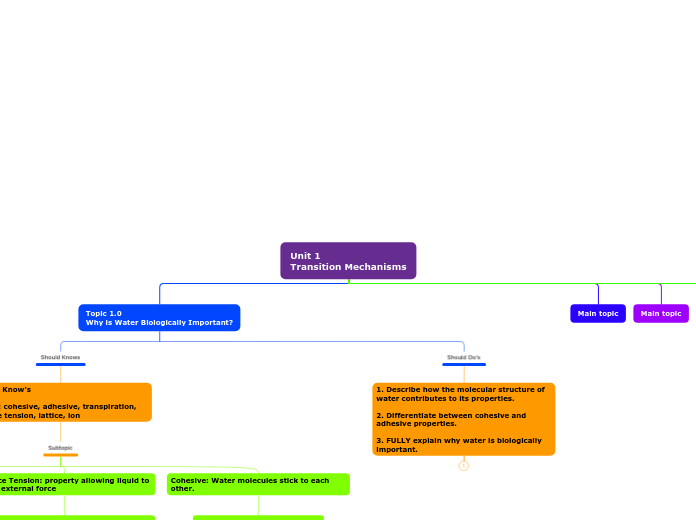
Topic 1.0
Why is Water Biologically Important?
Should Do's
1. Describe how the molecular structure of water contributes to its properties.
2. Differentiate between cohesive and adhesive properties.
3. FULLY explain why water is biologically important.
1. the waters role as a solvent helps cells transport
2. Cohesive is when water molecules stick with each other, while adhesive is when water molecules stick to other substances, like in the example of Xylem.
3. universal solvent, high heat compacity ( maintains constant temperature ) , a polar covalent molecule that is both adhesive and cohesive and forms a lattice
Should Knows
Should Know's
Define: cohesive, adhesive, transpiration, surface tension, lattice, ion
Subtopic
Cohesive: Water molecules stick to each other.
Ion: a charged atom or particle
Surface Tension: property allowing liquid to resist external force
Lattice: A pattern created by molecules in a crystal
Adhesive: water molecules stick to other substances
Transpiration: Moving water out of the plant