av Youssef Morsy 10 måneder siden
243
SCH3U Final Summative Concept Map
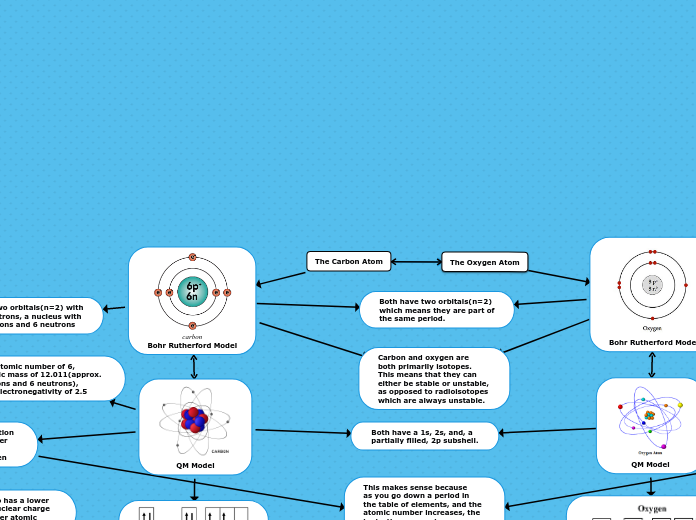
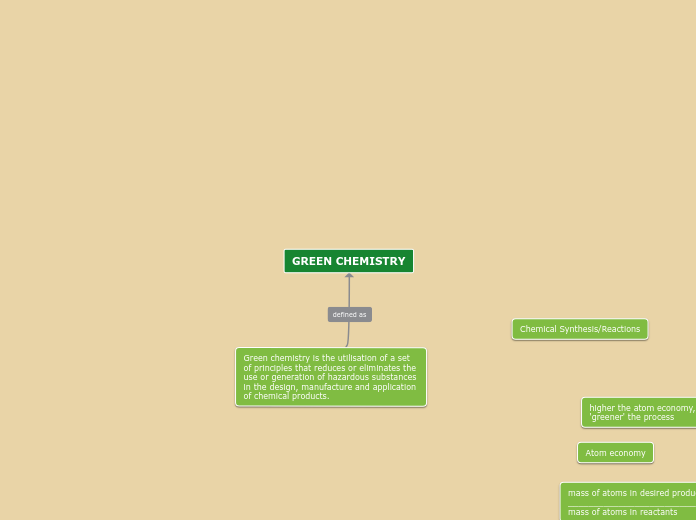
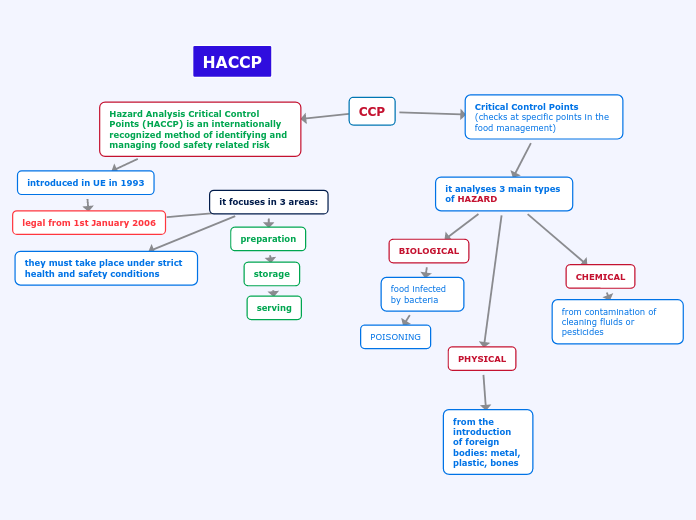
The text discusses the understanding and application of various chemistry concepts. It highlights the law of definite proportions, periodic trends such as ionization energy and electron affinity, and the ability to predict elements based on these trends.