Transfer of Thermal Energy
Applications of Thermal Energy Transfer
Common applications of radiation
Vacuum flasks
prevents heat from exiting or entering
Greenhouses
Teapots
shiny teapots can keep tea warm longer than black teapots
Common applications of convection
Refrigerators
Air-conditioners
Household hot water system
Heating water in electric kettles
Common applications of conduction
Uses of bad conductors of heat (insulators)
fiberglass, felt and expanded polystyrene foam
Woolen clothes
Wooden ladles
Sawdust
Table mats- made of cork
Uses of good conductors of heat
Heat exchanges
Soldering iron rods
Cooking utenseils
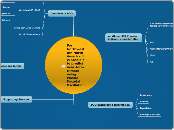
Causes of transfer of thermal energy
no transfer of heat at thermal equilibrium
flows from a region of higher temperature to a region of lower temperature
difference in temperature
How thermal energy is transferred
An object with more heat than another does not necessary means it has a higher temperature than the other object
does not require a medium
Can happen in vacuum
Requires no medium
Due to infra-red radiation
Subtopic
takes place in only liquid and gas
Can happen in liquid and gas
Due to changes in density
takes place in solid, liquid, gas
Cannot happen in vacuum
Requires a medium
Can happen in solid, liquid, gas
Due to vibration of particles
Radiation
infrared radiation is absorbed by all objects and surfaces
An object absorbs heat when its temperature is lower than the surrounding
An object emits heat when its temperature is higher than its surrounding
Poor emitter is also a poor absorber
Good emitter is also a good absorber
causes temperature rise
The hotter the object, the greater the radiant heat emitted
Factors affecting rate of infrared radiation
Surface area
Surface temperature
Colour and texture of the surface
thermal energy from infrared waves is called radiant heat
All object radiate heat in the form of infrared radiation as they are above 0 kelvin
Continual emission of infrared waves from the surface of all bodies, transmitted without the aid of a medium
Convection
Air-con {convection current}
Warm air reaches the air-con gets cooled
Warm air at the bottom which is less dense rises
D=m/v , hence the density of the air increases and it sinks to the bottom
The air contracts and its volume is decreased
Air near the air-con is cooled
Kettle water heater
Cold water gets heated up by the water
colder water at the top is denser, hence it sinks
density decreases, hence it rises
volume increases
Water at the base gets heated up
Transfer of heat energy in fluids due to the difference in density
Particles do not expand/contract, it is the substance/object that expands/contracts.
Conduction
Conduction in liquids and gases
Hence, air is poor conductorof heat compared to water, which is in turn poor compared to solids
liquid particles are further apart and collisions of particles are less frequent and even lesser in gases
thus, transfer of kinetic energy from fast moving molecules to neighbouring molecules is slower
process of conduction is inefficient
When thermal energy is supplied to one end of a rod, the particles at the hot end vibrate vigorously
These particles collide with neighbouring particles, making them vibrate as well
Metals contain free electrons which move randomly between the atoms and molecules
Non metals do not have free electrons
Conductors and insulators have different mechanisms
Insulators
No free electrons
In insulators, the transfer of thermal energy is solely the results of vibrating atoms and molecules
No free electrons
Good conductors
Fast moving electrons then diffuse into cooler parts of metals
Free electrons gain kinetic energy and move faster
In metals, another much faster mechanism of thermal energy takes place at the same time (free electron diffusion)