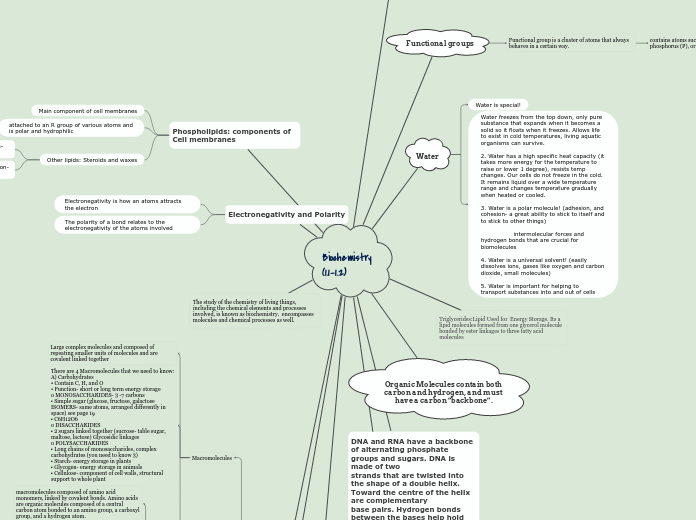
Biochemistry (1.1-1.2)
This template is designed to help you with studying for an exam or qualification. The ideas behind it are described in this article together with tips and suggestions for study techniques.
Begin by typing in the name of the subject that you are studying.
Intramolecular
Two types of intermolecular reactions are hydrogen bonding and hydrophobic reactions
Hydrophilic this exists in an aqueous environment, their polar molecules have a natural tendency to form hydrogen bonds. (Water-loving)
Hydrogen bonding is a water molecule that is polar because of its O-H bonds
The forces are weaker than intramolecular forces
Intramolecular bonds are the strong bonds that hold the atoms in a given molecule together.
Intermolecular
Hydrophobic non-polar molecules do not form hydrogen bonds with water (water-fearing)
Tend to clump together, away from water molecules. They do not mix
The breaking and re-forming of bonds in DNA play an important role in how DNA functions in the cell.
Intermolecular forces are forces that exist between molecules.
Molecules
The rest of the map (outside the Dashboard and baseline) is for Mind Mapping or Concept Mapping your notes and summaries. If you use multiple maps, make links to them from this one.
Micromolecules
small molecules essential to life
WATER- waste product of cellular respiration, needed for photosynthesis, POLAR
OXYGEN- cells use to release energy from food (cellular respiration)
CARBON DIOXIDE- waste product of cellular respiration and needed for photosynthesis.
Macromolecules
Protein
Denaturation of proteins
Extreme hot or cold (or exposure to certain chemicals) can cause proteins to completely unfold in a process called denaturation.
Denaturation occurs when the bonding between R groups is disturbed and intermolecular bonds break, potentially affecting the secondary, tertiary, and quaternary structures.
Once a protein loses its normal three-dimensional shape, it can no longer perform its usual function.
polypeptides are polymers composed of many amino acids linked by covalent bonds, including proteins.
catalyzing chemical reactions
• providing structural support
• transporting substances
• enabling organisms to move
• regulating cellular processes (hormones)
• providing defence from disease (antibodies)
macromolecules composed of amino acid monomers, linked by covalent bonds. Amino acids are organic molecules composed of a central carbon atom bonded to an amino group, a carboxyl group, and a hydrogen atom.
There are 4 Macromolecules that we need to know:
A) Carbohydrates
• Contain C, H, and O
• Function- short or long term energy storage
o MONOSACCHARIDES- 3 -7 carbons
▪ Simple sugar (glucose, fructose, galactose ISOMERS- same atoms, arranged differently in space) see page 19
▪ C6H12O6
o DISACCHARIDES
▪ 2 sugars linked together (sucrose- table sugar, maltose, lactose) Glycosidic linkages
o POLYSACCHARIDES
▪ Long chains of monosaccharides, complex carbohydrates (you need to know 3)
▪ Starch- energy storage in plants
▪ Glycogen- energy storage in animals
▪ Cellulose- component of cell walls, structural support to whole plant
Large complex molecules and composed of repeating smaller units of molecules and are covalent linked together
The study of the chemistry of living things, including the chemical elements and processes involved, is known as biochemistry. encompasses molecules and chemical processes as well.
Electronegativity and Polarity
The polarity of a bond relates to the electronegativity of the atoms involved
Electronegativity is how an atoms attracts the electron
Phospholipids: components of Cell membranes
Other lipids: Steroids and waxes
Waxes are lipids composed of long, carbon-based chains.
Lipids composed of four attached carbon-based rings
attached to an R group of various atoms and is polar and hydrophilic
Main component of cell membranes
The functional group or groups on a molecule determine the properties of the molecule. Important functional groups in biological molecules include hydroxyl, carboxyl, carbonyl, amino, sulfhydryl, and phosphate groups.
DNA and RNA have a backbone of alternating phosphate groups and sugars. DNA is made of two
strands that are twisted into the shape of a double helix. Toward the centre of the helix are complementary
base pairs. Hydrogen bonds between the bases help hold the two strands together. Three hydrogen bonds occur between G and C base pairs, while two hydrogen bonds occur between A and T base pairs.
Since RNA exists as a single-stranded molecule, it does not occur as a double helix.
Organic Molecules contain both carbon and hydrogen, and must have a carbon “backbone”.
Triglycerides:Lipid Used for Energy Storage. Its a lipid molecules formed from one glycerol molecule bonded by ester linkages to three fatty acid molecules
Water
Water freezes from the top down, only pure substance that expands when it becomes a solid so it floats when it freezes. Allows life to exist in cold temperatures, living aquatic organisms can survive.
2. Water has a high specific heat capacity (it takes more energy for the temperature to raise or lower 1 degree), resists temp changes. Our cells do not freeze in the cold. It remains liquid over a wide temperature range and changes temperature gradually when heated or cooled.
3. Water is a polar molecule! (adhesion, and cohesion- a great ability to stick to itself and to stick to other things)
intermolecular forces and hydrogen bonds that are crucial for biomolecules
4. Water is a universal solvent! (easily dissolves ions, gases like oxygen and carbon dioxide, small molecules)
5. Water is important for helping to transport substances into and out of cells
Water is special!
Functional groups
Add resources that will help your studies in the topics below.
Functional group is a cluster of atoms that always behaves in a certain way.
If you have been given a reading list, map it out below, and add any other relevant publications such as professional journals, published papers, conference proceedings, webinars, and other books.
contains atoms such a oxygen (O), nitrogen (N) , phosphorus (P), or sulfur (S)
Publication
Carbon
The 'dashboard' topic holds useful information to help organise your studies. It is separate from the notes themselves.
Carbohydrates are biological molecules that contain carbon, hydrogen, and oxygen (sugars and starches) is (CH2O
What are your purposes for this study? What will you be able to do when it is completed successfully?
Disaccharides are composed of two monosaccharides joined by a glycosidic linkage (sucrose and lactose)
monosaccharide composed of a single,carbon-based monomer structure. Simple sugars woth 3 to 7 carbon atoms. (glucose , fructose, and galactose) that are isomers (molecules having the same molecular formula but different structures)
Polysaccharides are composed of two monosaccharides joined by a glycosidic linkage. (sucrose and lactose)
Purpose
•glycogen - glucose as stored by animals. This form is easily broken down due to the numerous branching side chains.
• starch - glucose as stored by plants. Starch has fewer branching chains than glycogen and is broken down less easily.
• cellulose - provides structural support in plant cell walls. Humans lack the enzymes to break down cellulose.
polar
All living things have some kind of carbon, and carbon is the main building block of macromolecules like proteins, lipids, nucleic acids, and carbohydrates. This leaf contains carbon in a variety of forms, including the cellulose that gives the leaf its structure and the chlorophyll that gives the leaf its green colour.
Map out the syllabus for this subject in the tree below. If you have not received a syllabus, make sure you get a copy.
Although it can make so many various types of bonds and create necessary compounds, carbon is the most crucial element for all living things.
What are the deadlines for your studies? Add information about exam dates, new projects to start or changes to make.
"Backbone of life "
What are your goals in studying this subject? Are you aiming to pass an exam or gain a qualification? Write it down with target grades if they are important to you.