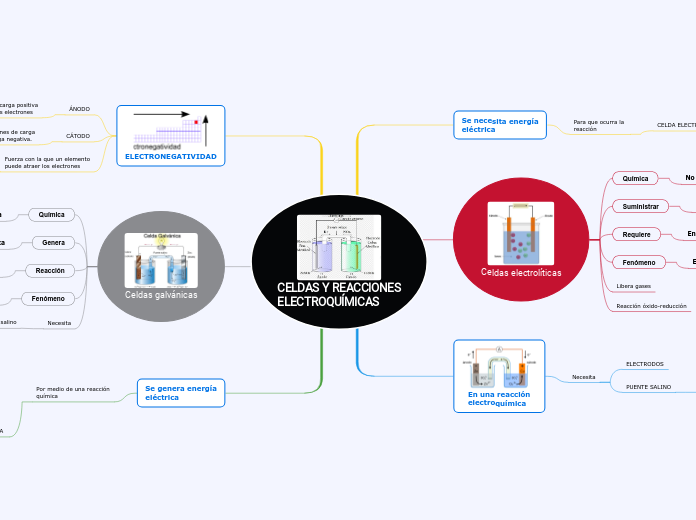
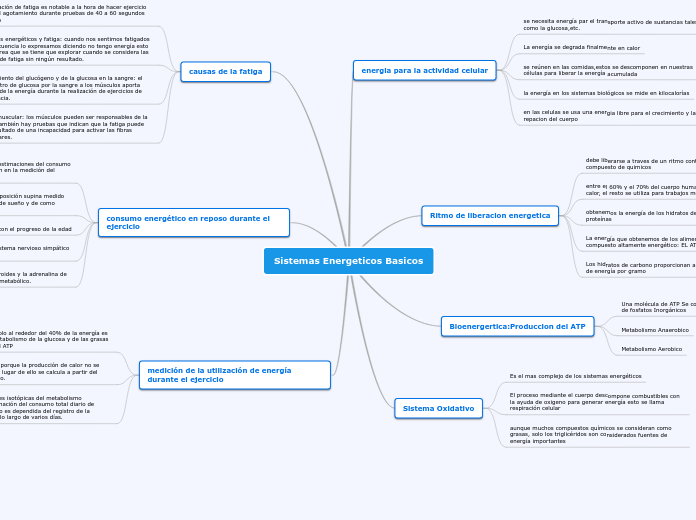
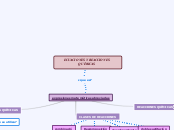
CELDAS Y REACCIONES
ELECTROQUÍMICAS
The Solar System is the gravitationally bound system of the Sun and the objects that orbit it, either directly or indirectly. Of the objects that orbit the Sun directly, the largest are the eight planets, with the remainder being smaller objects, the dwarf planets, and small Solar System bodies.
Se genera energía
eléctrica
Por medio de una reacción
química
CELDA ELECTROOUÍMICA
Celdas galvánicas
Saturn is known most for its rings.
Galileo Galilei first thought it was an object with three parts: a planet and two large moons on either side.
Not knowing he was seeing a planet with rings, the stumped astronomer entered a small drawing — a symbol with one large circle and two smaller ones — in his notebook.
The rings are made of ice and rock and scientists are not yet sure how they formed. The gaseous planet is mostly hydrogen and helium.
Puente salino
Saturn has over 150 moons and satellites. However, of these vast numbers of moons, only 62 are known and confirmed as moons.
Name at least 5 of these moons.
De corrosión
Reacción
How long does it take for Saturn to go around the sun?
óxido - reducción
Genera
A planet's day is the time it takes the planet to rotate or spin once on its axis.
Write down Saturn's day measured in Earth days.
Espontánea
ELECTRONEGATIVIDAD
Fuerza con la que un elemento
puede atraer los electrones
DE IZQUIERDA A DERECHA
CÁTODO
Atrae a los iones de carga
positiva. Carga negativa.
REACCIÓN DE REDUCCIÓN
ÁNODO
Queda con carga positiva
al perder los electrones
REACCIÓN DE OXIDACIÓN
En una reacción
electroquímica
Necesita
PUENTE SALINO
Para el paso de energía
Separación de iones
-->IONIZACIÓN
ELECTRODOS
Celdas electrolíticas
Mars is a cold, desert-like place covered in dust. This dust is made of iron oxides, giving the planet its iconic red hue.
Mars shares similarities with Earth: It is rocky, has mountains, valleys and canyons, and storm systems ranging from localized tornado-like dust devils to planet-engulfing dust storms.
Reacción óxido-reducción
Libera gases
Fenómeno
Mars has two small moons.
Name these moons.
Electrolisis
Requiere
How long does it take for Mars to go around the sun?
Energía externa
Suministrar
A planet's day is the time it takes the planet to rotate or spin once on its axis.
Write down Mars's day measured in Earth days.
Energía eléctrica
Química
Our Solar System has eight “official” planets which orbit the Sun.
Each planet is at a different distance from the sun. Name its position.
No Espontánea
Se necesita energía
eléctrica
Para que ocurra la
reacción
CELDA ELECTROLÍTICA