Chapter 4: Proteins
Fibrous Proteins
Structure of Collagen
Significant Gly, Pro content
Skin, Tendons, Cartilage, Bones
Structure of Silk
Fibroin consists of layers of antiparallel B-sheets rich in Ala and Gly residues
The fibers in silk cloth and in a spider web are made up of the protein fibroin
Chemical Reduction of the Cystine
Reduce --> Curl --> Oxidize
Crosslinking Keratin Fibrils: to curl or not to curl
Structure of Hair
Keratin
Significant Cys content
Hair, Claws, Outer Epidermal Layer
Not Globular
Hydrophilic, but not soluble
Very tough- occur in skin, nails, cocoons
Long rope or sheet-like structures
Levels of Protein Structure
Quartenary Structure
Assembled subunits
Interactions between polypeptide chains
Tertiary Structure
Polypeptide chain
Independently Folding Regions (domains)
Distinct Interactions
Secondary Structure
B-turn
Type I occur 2x more frequently than Type II
Hydrogen bond between the peptide bonds of 1st and 4th residues
Type II have Gly in 3rd position
Glycine: small side chain allows for tight corners
Proline are preferred in 2nd position
Proline: conformationally restricted with fixed phi angle keeps turn rigid
B- sheet
Extended chains
Peptide chains align side-by-side
Parallel and Antiparallel
Amide groups Hydrogen bond
Extended peptide anions- pleated geometry
Planar peptide bonds are in the pleat
R-groups alternate (up, down, up, down..)
At Carbons are the apices
a- helix
Geometry
This allows for helix faces
Charged: helix with a string of similarly charged residues
Amphipathic: hydrophobic face and hydrophilic face
There are 3.6 residues per turn- roughly every 3rd to 4th residue will be on same face
All side chains point outward from core
Very compact structure
Stabilized by intramolecular Hydrogen bonds between peptide bonds
Peptide "backbone" is the core of the coil
R-groups protrude outwards
Right-handed twist
Chain is coiled like a spring
Local Interactions
Primary Structure
Ramachandran plot
Primary Structure dictates fold...but it's complicated
Proteins may fold in one environment, then move to another environment
Other proteins can help fold the protein
Proteins start to fold before they are completely made
Amino Acid Residue
Protein folding
Assume the native state is folded and is the most stable state
Why is the Native state more stable?
Denatured state is less stable
Noncovalent forces stabilize the native state relative to the unfolded state
Hydrophobic Interactions: Drive burial of hydrophobics in the protein interior
London Dispersion Forces: Between hydrophobic residues in the protein interior
Ionic Interactions: Acidic and Basic groups (e.g. Asp-Lys)
Hydrogen bonding: Inter- and Intramolecular Hydrogen bonds
Revised
Changing primary structure can change the energetics of folded state or the folding energy landscape
Some proteins require chaperones to fold
They help overcome energy barriers or alter salvation to change the folding landscape
Protein dynamics may play a crucial role in functions e.g. Histones!
Folded proteins may have many conformations. Proteins move!
"Native" structure is not always folded. Intrinsically Disordered Proteins!
Not random
Molten globule by a spontaneous hydrophobic collapse first, followed by formation of secondary structures
Hierarchic process in which local secondary structures form first, followed by hydrophobic collapse
Fast process
Protein Stability
Hydrophobic collapse drives the energetics of most protein folding
Denaturants
Chemicals
Guanidine
Urea
Heat
Denaturation
Monitor a signal
Enthalpy
Spectral
Fluorescense
Circular Dichroism
Unfolding is cooperative
Protein Structure Determination
Circular Dichroism Spectroscopy
Chiral Centers
Secondary structures are unique arrangements of chiral centers leading to unique spectra
Unique absorption of circularly polarized light
Monitors protein structure by recognizing secondary structures
Differential absorption of circularly polarized light
NMR Spectroscopy
No direct image of protein is obtained, just a series of spatical constraints
Complicated
Only small proteins can be studied
Dynamics of protein-ligand interactions
Motional dynamics of whole molecule
Protein is in solution
Rate of recovery is related to the environment of the nucleus
After pulse- recover to original angle
Pulse of radio frequency resonant to precession frequency of nucleus: nucleus will flip to precess at different angle
In high strength magnetic field- unpaired nuclei process
Track nuclear spin on unpaired nuclei (1H, 13C (6 protons and 7 neuts), 19F (9 prots and 10 neuts))
X-Ray Crystallography
Disadvantages
Solution conditions are not "native"
Crystal contacts can distort important regions of proteins
Structure is static
Proteins must crystallize; not aqueous structure
Advantages
Large proteins and protein complexes
Very High resolution - 1.5 A
Diffraction pattern is used to construct a
3D arrangement of atoms
X-rays diffract off of electron clouds of C,N,S,O-
but usually not H
Crystals are blasted with X-rays
Proteins are crystallized into 3-D lattices
Chapter 3:
Amino Acids
Methods of Separation
Isoelectric Focusing
Proteins migrate to pH=pI
Ampholytes establish stable pH gradient
Isoelectric Point
Estimating the Molecular Weight of a Protein by Electrophoresis
Migration depends on Charge and Size
Migration of ions in electric field
Chromatography
Affinity Chromatography
PolyT
Elution: Free Thymine
Immobilized PolyT
Purpose: mRNA purification
His Tag
Elution: High imidazole in elution buffer
Imidazole chelates Ni 2+ on column, so His-tagged protein sticks on column
Proteins are engineered to have a His6 sequence
Ni 2+ is immobilized on column
Engineered into gene sequence
Ion Exchange Chromatography
Cation Exchange
Wash off impurities (-) and neutral impurities
Choose buffer so analyte is (+) charged
Cations stick to (-) charged column
Anion Exchange
Elute analyte with High salt or basic conditions (deprotonates analyte)
Wash off impurities (+) and neutral impurities
Equilibrate column then adsorb analyte to column
Choose buffer so analyte is (-) charged
Anion stick to (+) charged column
Size Exclusion Chromatography
Separates based on size
Exchanges buffer components between sample matrix and column buffer
No adhesion to the stationary phase
Large molecules are more often in flow path, so they elute first
Small molecules explore internal space and are out of flow path
Porous particles
Centrifugation
Separate Soluble from Insoluble
Membrane Components
Soluble Proteins
Separate Mitochondria
Isopycnic Centrifugation
Takes a sample and the more dense component settles to the botton while the least dense component floats in the center
Separate Organelles
Differential Centrifugation
Left with pellets containing ribosomes and large macromolecules
Keeps breaking up the materials so that they become soluble proteins
Spins very fast and separates organelles
Disulfide Bonds
Covalent link between side chains
Polar, Charged Amino Acids
Acidic
Glutamate
Aspartate
Basic
Histidine
Arginine
Lysine
Polar, Uncharged Amino Acids
Glutamine
Asparagine
Cysteine
Threonine
Serine
Aromatic Amino Acids
Tryptophan
Tyrosine
Phenylalanine
Hydrophobic Amino Acids (Aliphatic)
Methionine
Isoleucine
Leucine
Valine
Proline
Alanine
Glycine
Ionizable Groups on Amino Acids (pKa)
Arginine : 12
Lysine : 10
Histidine : 6
Glutamate : 4
Aspartate : 4
a-amino : 10
a-carboxylate : 2
Biological View of Chemical Bonding
Hydrophobic Interactions
Water forms cages around hydrophobics (antiparallel orientation of water dipoles); Very LOW Entropy of solvent
Decrease in entropy of water drives the burial of hydrophobic surface area
Hydrophobic groups condense. Preleases trapped water to bulk
Micelle forms. Water released to bulk solvent
Micelle formation: High Entropy of solvent
Van der Waals Interactions
Dipole-Dipole Interactions
London Dispersion Factors
Biomolecule
Fatty Acid
Micelle
Globular Protein
Hydrophobic amino acids interact in center of proteins
Hydrophobic tails in center of the micelle interact with London Dispersion Forces
Always attractive interactions
Weak, local interactions between the fluffy, deformable electron clouds of hydrophobic groups
Occur in the absence of water
Favorable Interactions between hydrophobic molecules
Ionic Interactions
Biomolecules
Critical for folding and function of biological molecules
Like charges attract and opposite charges repel
Charged side chains and Nitrogen and Carbon termini
Solubility of NaCl
Water is dipole
Orients Hydrogen toward anions and Oxygen toward cations
Bent is weaker Hydrogen Bond
Linear is stronger Hydrogen Bond
Covalent Bonds
Bond dissociation energy
Difinition
Life: A diverse collection of proteins, nucleic acids, lipids, and carbohydrates that undergo chemical reactions allowing for:
Use Energy from environment to do chemical work
Metabolism
Makes ATP and reduced conenzymes
Degradative
Oxidative
More bonds to Oxygen
Anabolism requires energy input
Reductive
More bonds to Hydrogen
Uses ATP and reduced coenzymes
Eat
Synthetic
Kinetics
Thermodynamics
Free Energy (delta G)
Excess Energy that can be
used as work or heat
Equilibrium
Dynamic interconversion of products
and reactants
"How far?"
Heat, work, and energy of chemical reactions
Catalysts: lower activation energy barrier
Enzymes Stabilize Transition
State conformation
Activation Energy (delta G+)
Energy required to overcome
the Activation Barrier
Activation Barrier
Determined by energy of
Transition State
Transition State:
Intermediate between
substrate and product
Unstable
Distinct conformation
Slow rate results from
high activation energy barrier
"How fast?"
Rate of Chemical Reaction
Equilibria
Precise self-replication and assembly
Evolution
Low Density of Ice
Ice cap on large or moving bodies of water
Temperature of water below ice is
buffered from subzero air
Water doesn't freeze through
High Specific Heat of water
Terrestrial Animals
Internal water buffers
extremes of temperature
Imprecise Self-replication
Cell Mitosis
Segregating subcellular structures
into daughter cells
Central Dogma
Replication, Transcription, Translation
Complexity and Organization
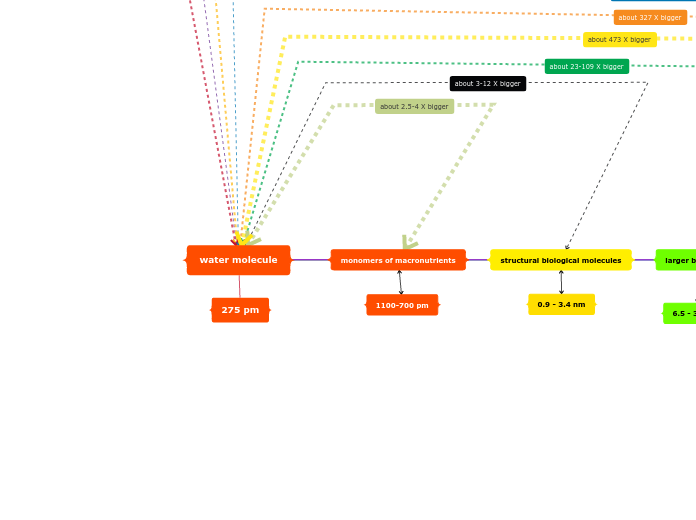
Structural Hierarchy: Molecules to Cells
Level 4: The cell and its organelles
Level 3: Supramolecular Complex
Cell wall
Plasma Membrane
Chromatin
Level 2: Macromolecule
Cell Wall --> Cellulose
Plasma Membrane --> Protein
Chromatin --> DNA
Level 1: Monomeric Units
Cellulose --> Sugars
Protein --> Amino Acids
DNA --> Nucleotides
Tissue Specific Expression of Genes
Genomic DNA
~3000 are expressed in all cells
20,000 to 24,000 genes
Microscopic Motion
Dynein
Macroscopic Motion
Muscle, Bone, Sinew
Biological Polymers
Separation from,
but response to environment
Compartmentalization
of functions
Selective Permeability
Membranes are Complex
Peripheral
Integral
Fluid Mosaic Model
Fat Soluble Materials
Signaling Lipids
Receptors
Membranes
Lipid barriers between
aqueous compartments
Phosphoglycerides
Phosphate
Glycerol
Water
Water as a Solvent
Noncovalent Bonds
Ionic Interaction
Hydrophobic Interaction
Van der Waals Interaction
Dipole-Dipole Interaction
Ion-dipole Interactions
Hydrogen Bonds
Key to Interactions of
Biological Molecules
Good Solvent, Bad Solvent
Ice Floats
Polar, Dipole
Biological Molecules
and Digestion
Biological Macromolecules
Polymers
Cholesterol
Sphingolipids
Phosphoacylglycerol
Lard - TAGs in animals - saturated FAs
Oils - TAGs in plants - unsaturated FAs
Storage in Adipose
FAs esterified to glycerol
Fatty Acids
Polysaccharides
hemiacetals and acetals
Glycosidic bonds
Monosaccharides
Nucleic Acids
Phosphodiester
Ribophosphate backbone
Nucleotides
Amino Acids
R= side chains
20 common acids
folded complex structures
3 or 4 degrees structures
Chaos to order to chaos
Complex Molecules
Lipids
Glycogen
Work in Body
Coordinated synthesis and Degradation
Dysequilibrium
Muscle Contraction
Plants
Photosynthesis
Glucose
Starch
Ate and Digested
Anabolism
Catabolism
Animals
Organs of Digestive System
Key Concepts
Pancreas
Synthesis of digestive enzymes
Pro-peptidases/ Peptidases
Pro-elastase/ Elastase
Chymotrypsinogen/ Chymotrypsin
Trypsinogen/ Trypsin
Triacyglycerol
Pancreatic Lipase
Hormones
Bile acid synethesis
Bile Salts
Enzymer
Harvesting
Small Intestine
Adsorption of small molecules
Transporters
Intestinal lining
Digestion of Fats
Pancreatic Lipase (+Colipase)
Bile Acids
Liver
Pancreatic Enzymes
Zymogens
Neutralization
Stomach
Zymogen/Protease
Activation by acid
Protease- Enzyme that hydrolyses peptide bonds (Pepsin)
Zymogen- Inactive precursor protease (Pepsinogen)
Hydrolyze peptide bonds
Pepsinogen/Pepsin
Acidic Conditions
Strong Acid
Activates pepsinogen to pepsin in stomach
Unfolds proteins
Acidifies chewed food entering stomach
Completely dissociates
Cells in stomach lining secrete HCl
Stomach lining epithelium is protected from acid by mucins
Secreted after meal is eaten
Peptide bonds
Amides
Stable polymers folded into complex structures
Purpose
Digestion of Proteins
Homogenization
Acid
Mouth
Saliva
pH
Enzymes
Amylase
Proteins
Mucopolysaccharides
Antimicrobials
Enzymes that degrade or poison microbes
Teeth
Chewing
Hydrolysis
Waste Disposal
Indigestible
Low surface Area foods
cellulose
Digestible
catabolism to generate ATP
transport to tissues
enzymes convert to complex to simple
Why do we eat so often?
It takes lots of food to keep us warm
Why do we eat?
ATP for maintaining our bodies
Raw materials for anabolism
What do we eat?
Proteins, Carbohydrates, and lipids
Chapter 2:
Acid-Base Chemistry
Strong Acids and Strong Bases
Strong acid + Strong Both = water + salt
Strong Bases completely dissociate in water
Strong Acids completely dissociate in water
Not an equilibrium
Biochemistry
Chapter 1: Life