CLOROX BLEACH
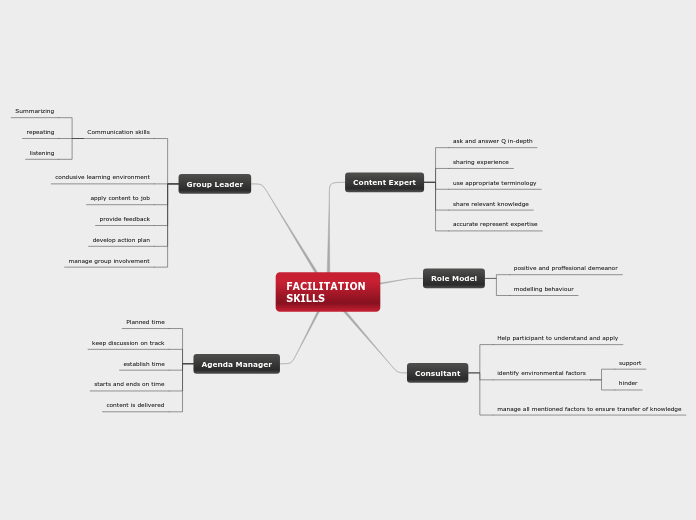
Effects on Enviorment
WATER-Bleach gets released into our water sytems
it them reacts with other elements and minerals to form dangerous toxins like including dioxins, furams and PCDDs.
WILDLIFE- Dioxins caused by bleach were responsible for the decimation of the bald eagle population during the mid-20th century, and continue to reduce the number of fish and bird species near the Great Lakes. The World Wildlife Fund also warns that these chlorine by-products can cause mutations, sterility and even extinction in wildlife species.One of the most significant problems with chlorine bleach is its persistence. Even low levels released into air and water supplies will accumulate over time, and may lead to long-term health concerns
AIR-Airborne chlorine bleach by-products eventually reach Earth's atmosphere and the ozone layer. Chlorine bleach is linked to ozone depletion, which has far-reaching environmental effects in terms of global warming.According to the Reach for Unbleached Foundation, bleach related toxins can cause severe long- and short-term respiratory irritation upon inhalation. They may also contribute to problems with the immune system, blood and heart.
WARNINGS!
DANGER: CORROSIVE
this product causes burns,dangerous fumes are caused when mixed with other products. Do not breathe this product in. Do not swallow. Do not get in eyes or on skin or clothing. HANDLE WITH CARE!!!!!!
Ways to lessen harmfullness of product
ALTERNATIVE GREEN PRODUCTS
In house hold use dilute product as much as possible
ALTERNATE PRODUCTS
-Vinigar
-Lemon and baking soda
-Peroxide
How to use safley
-Where mask
-safety glasses
-rubber gloves
-usesw in well ventilated areas
What is and how does
SODIUM HYPOCHLORITE
Work !
HYPOCHLORITE is produced by the following reaction
cl2 +2NOH + ______NaOCl + NaCl + NaCl + H2O Sodium Hypochlorite is a compound that is used to effectively dissinfect and purify on large or small scale.
Sodium hypochlorite dissenfects by quickly reacting with microbial cells to denature and destroy many pathogens. NaCl reacts with the microbes heat shock protiens and pretty much turns them into clumps. Much like you would boil an egg.
NaCl
Name of Active Ingrediant
Sodium Hypochlorite
makes up 5.5% of product.
Other ingrediants include:
Sodium Chloride,Sodium Hydroxide,
Sodium Carbonate and Sodium polyacrylate
Primary purpose
kills bacteria found in homes and offices. used to dissinfect hardnon-porous surfaces such as toilets sinks, floors , countertops, washable kitchen surfaces such as refrigerators and other appliances. Bleach kill pathogens and microbes on a very broadspectrum.