por Trudy Kwek 13 anos atrás
3139
Elements, Compunds & Mixtures
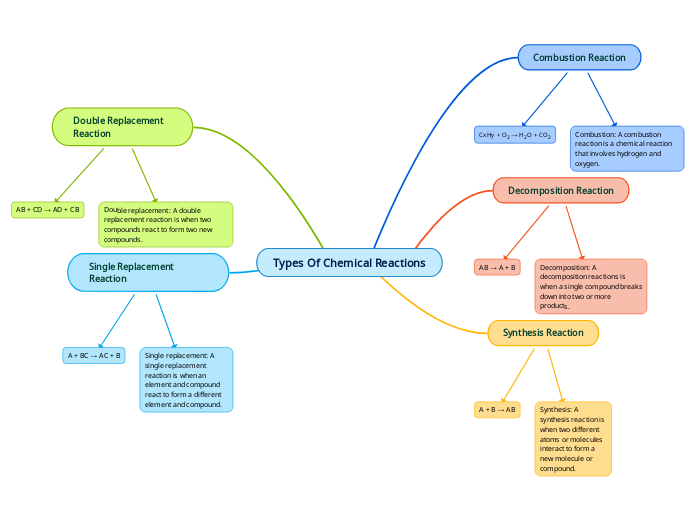
Substances can be categorized into elements, compounds, and mixtures, each with distinct characteristics. Elements are pure substances that cannot be broken down into simpler substances by chemical means.