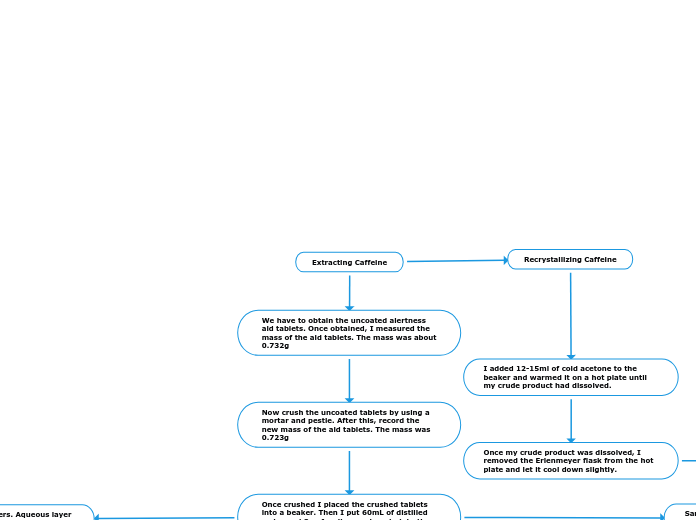
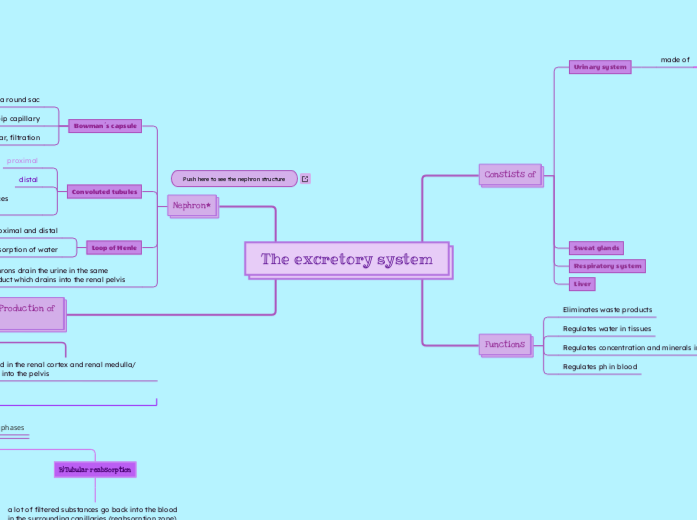
Extracting Caffeine
We have to obtain the uncoated alertness aid tablets. Once obtained, I measured the mass of the aid tablets. The mass was about 0.732g
Now crush the uncoated tablets by using a mortar and pestle. After this, record the new mass of the aid tablets. The mass was 0.723g
Once crushed I placed the crushed tablets into a beaker. Then I put 60mL of distilled water and 8g of sodium carbonate into the beaker.
Sample with tablet binder material and the undissolved impurities.
Next I quickly (but carefully) filtered the alertness aid pill mixture into a separatory funnel. Then I rinsed the remaining residue in the glass with distilled water.
At this point I allowed the aqueous filtrate to cool down to room temperature. This is because when adding dichloromethane to a hot solution can be dangerous.
Once it was cooled down to room temperature, I proceeded to add 7ml of the dichloromethane. Due to density differences, the aqueous layer was on the bottom while the caffeine (mixed with the dichloromethane) was on the top.
I then placed a stopper on the neck of the separatory funnel and started to shake gently. I inverted the funnel while simultaneously holding the stopper in the neck, and vented the separatory funnel through the stopcock. After this I obtain my two phases. The aqueous phase and the organic phase.
I had drained the organic layer into the correctly labeled Erlenmeyer flask.
This process was repeated twice (three times in total). This is because it helps in getting everything in the organic layer collected.
After all the extracts are collected. I proceeded to add certain amounts of drying agents. I let it sit in the flask for about ten minutes slowly adding the drying agent.
I carefully decanted the dried dichloromethane in a small 50mLbeaker.
I gently let the dichloromethane evaporate the solvent away in the hood.
Finally I measure the mass of the crude product which came to a mass of 0.454g
Aqueous layer
Organic Layer
There are two sample layers. Aqueous layer that contains the caffeine
Recrystallizing Caffeine
I added 12-15ml of cold acetone to the beaker and warmed it on a hot plate until my crude product had dissolved.
Once my crude product was dissolved, I removed the Erlenmeyer flask from the hot plate and let it cool down slightly.
Then I used a pipet and began adding petroleum ether in a titration fashion. After each addition of petroleum I swirled it around until I saw cloudiness in the solution.
I then recovered the recrystallized product via vacuum filtration using the Hirsch funnel.
I pulled some of the air through Hirsch funnel for a couple minutes. This is because it helps dry the solid.
Transferred the recrystallized product to a larger piece of filter paper and allowed it to dry for 20 minutes.
Finally once completely dried I measured the mass to be 0.410g and the melting point to be 231-234 degrees Celsius