por Cole Reis 4 anos atrás
468
Turning mercury into gold By Cole Reis
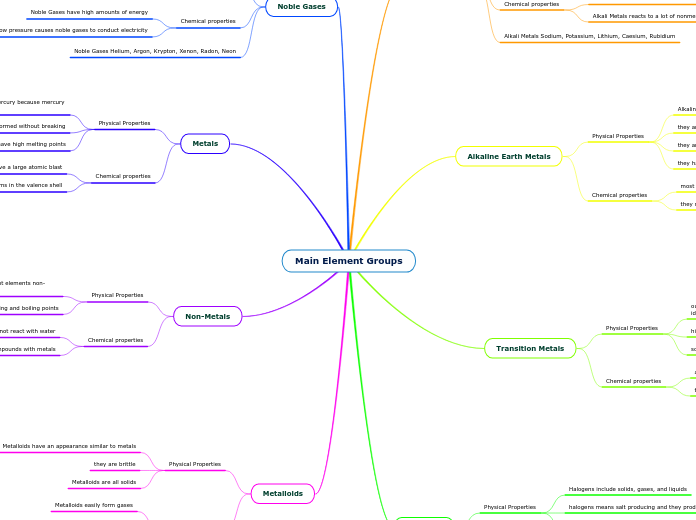
The text explores different categories of chemical elements, including nonmetals, noble gases, alkali metals, alkaline earth metals, halogens, and metalloids. Nonmetals are characterized by their low melting and boiling points, brittleness, and are not typically found in a free state in nature.