по LOGAN IRVINE 1 года назад
106
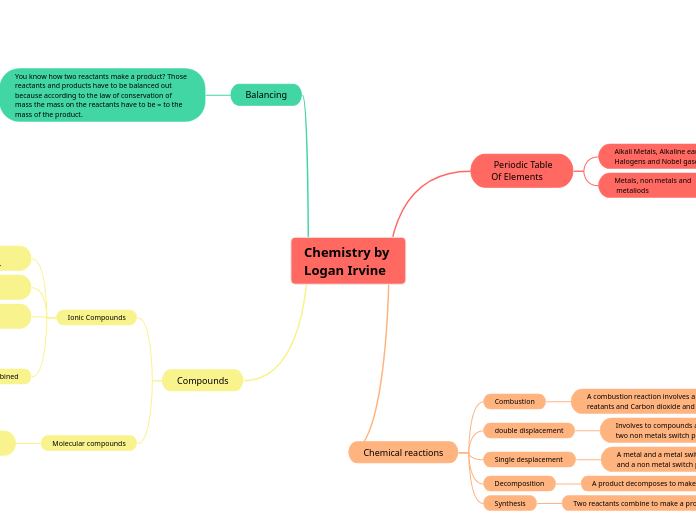
Chemistry by Logan Irvine
The text explores various fundamental concepts in chemistry, focusing on different types of chemical reactions, including decomposition, synthesis, double displacement, combustion, and single displacement.