av Rameen Sarwar för 8 månader sedan
72
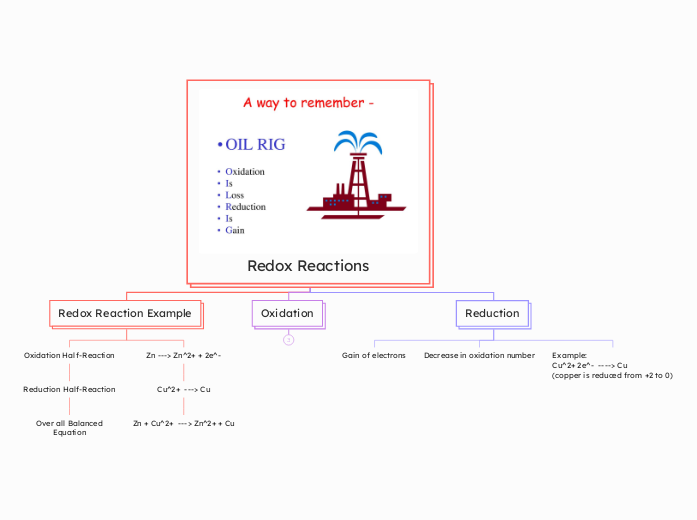
Redox Reactions
Redox reactions are fundamental chemical processes involving the transfer of electrons between two species. Oxidation is characterized by an increase in oxidation number and the loss of electrons, while reduction involves a gain of electrons and a decrease in oxidation number.