av Hartaj Gill för 2 årar sedan
228
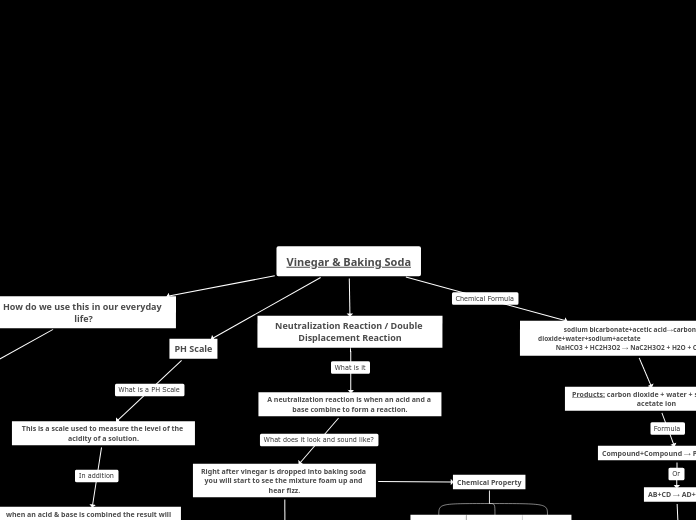
Vinegar & Baking Soda
When vinegar interacts with baking soda, it initiates a reaction that involves the exchange of molecules, leading to the formation of carbon dioxide, water, sodium ions, and acetate ions.
av Hartaj Gill för 2 årar sedan
228

Mer av detta
Chemical Property
Irreversible
Gives off light
All of these examples combined shows if a chemical reaction has a curd.
Gives off heat
The mixture will start to bubble up because carbon dioxide gas is released.
neutralization reactions are known for producing oxygen, salt and water.
AB+CD → AD+CB
This reaction has 2 parts. When the vinegar touches the baking soda it is a single displacement reaction. After bubbles start to form it then turns into a decomposition reaction(H2CO3 → H2O + CO2)