作者:Ayden koh 2 年以前
447
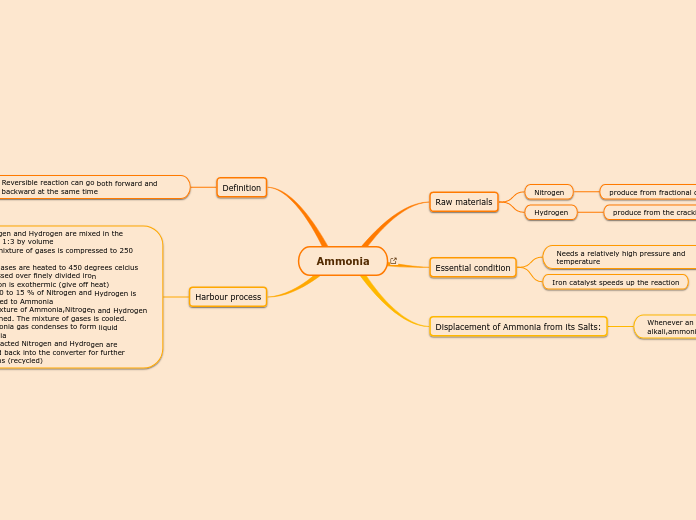
Ammonia Summary map
Ammonia is manufactured through a process that primarily involves nitrogen and hydrogen. These elements are derived from fractional distillation of liquid air and the cracking of petroleum, respectively.
作者:Ayden koh 2 年以前
447

更多类似内容
Check the link from this topic to learn more about global warming effects, then think about 7 ideas for preventing global warming.