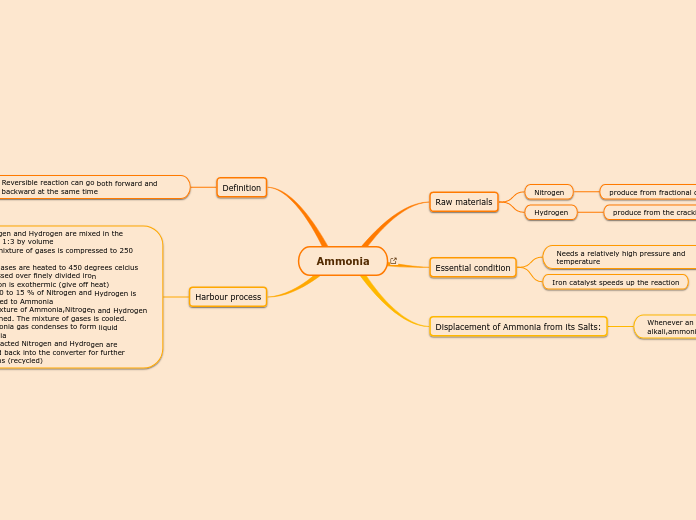
Ammonia
Check the link from this topic to learn more about global warming effects, then think about 7 ideas for preventing global warming.
Harbour process
1)Nitrogen and Hydrogen are mixed in the process 1:3 by volume
2)The mixture of gases is compressed to 250 atm.
3)The gases are heated to 450 degrees celcius and passed over finely divided iron
-Reaction is exothermic (give off heat)
-Only 10 to 15 % of Nitrogen and Hydrogen is converted to Ammonia
4) A mixture of Ammonia,Nitrogen and Hydrogen is obtained. The mixture of gases is cooled.
5)Ammonia gas condenses to form liquid Ammonia
6) Unreacted Nitrogen and Hydrogen are pumped back into the converter for further reactions (recycled)
Definition
Reversible reaction can go both forward and backward at the same time
The reaction between Nitrogen and Hydrogen is reversible process
Manufacture of Ammonia by Harbour process
using raw materials such as Nitrogen and Hydrogen
Displacement of Ammonia from its Salts:
Whenever an Ammonia salt is heated with an alkali,ammonia is displaced from the salt
Essential condition
Iron catalyst speeds up the reaction
Needs a relatively high pressure and temperature
Raw materials
Hydrogen
produce from the cracking of petroleum
Nitrogen
produce from fractional distillation of liquid air