作者:Angel Peng 3 年以前
178
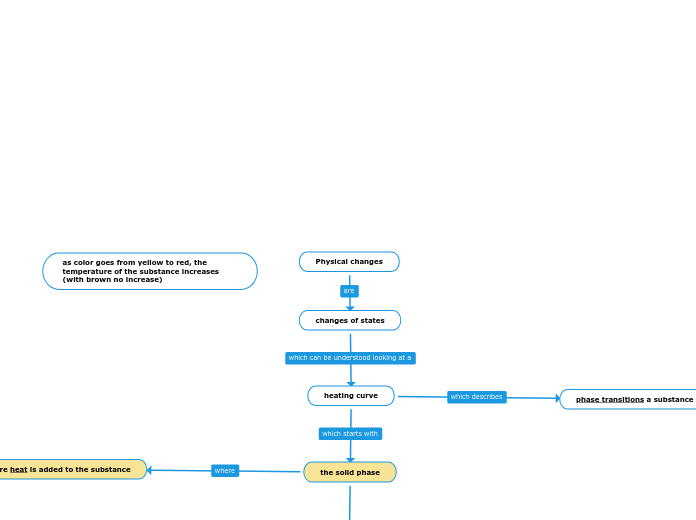
Physical changes
When a substance undergoes heating, it experiences a series of phase transitions. Initially, the substance is in the solid phase, where particles are closely packed and have limited movement.
作者:Angel Peng 3 年以前
178

更多类似内容
increase 1g of the substance by 1 degree celsius
raise 1g of the substance by 1 degree Celsius
specific heat capacity
heat (thermal energy in motion) is added
the melting phase
the liquid phase
the boiling phase
the gas phase
as more heat is added to the substance