von Merhamah Khan Vor 5 Jahren
3253
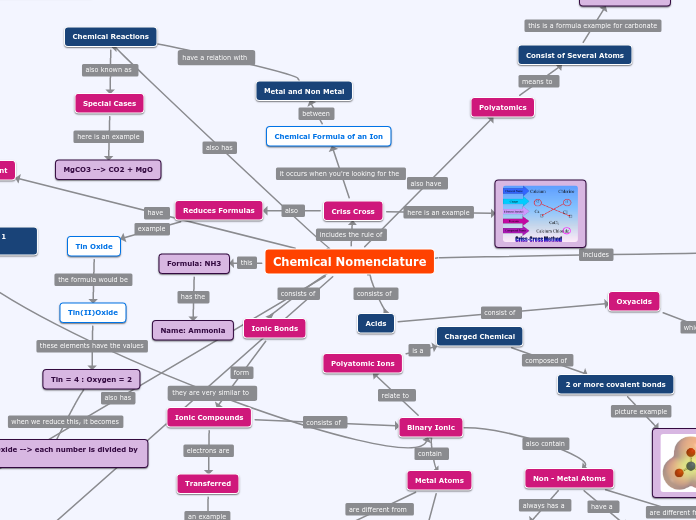
Chemical Nomenclature Concept Map
The subject focuses on chemical nomenclature, which involves naming compounds based on their structures and the types of bonds present. Diatomic molecules consist of only two atoms, while binary ionic compounds are formed through ionic bonds between metal and non-metal ions, resulting in positively charged cations and negatively charged anions.