By: Merhamah Khan
Examples
Connecting Words
Secondary Topic
Given Topic
Legend
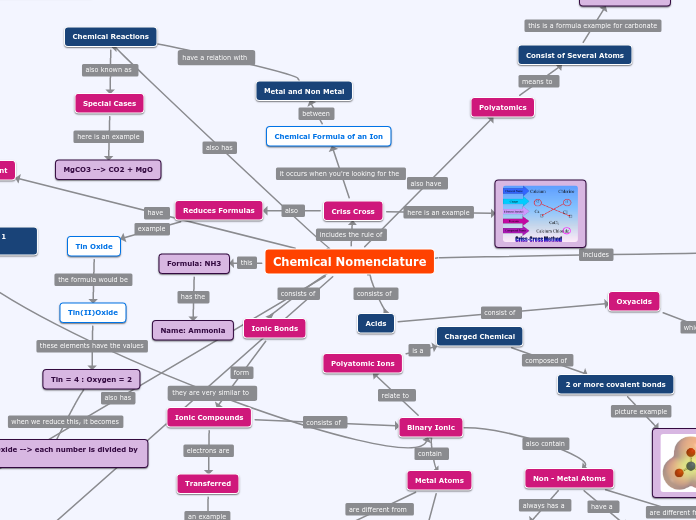
Chemical Nomenclature
Oxidation Charges
Degree of Oxidation of an Atom
Chemical Compound
Multivalent
The Positive Ion can have more than 1 charge
Copper, Gold, Iron, Lead
Polyatomics
Consist of Several Atoms
Diatomic
Molecules formed up from only 2 atoms
Chemical Reactions
Special Cases
MgCO3 --> CO2 + MgO
Criss Cross
Reduces Formulas
Tin Oxide
Tin(II)Oxide
Tin = 4 : Oxygen = 2
Tin (II)Oxide --> each number is divided by 2
Chemical Formula of an Ion
Metal and Non Metal
Formula: NH3
Name: Ammonia
Molecular Compounds
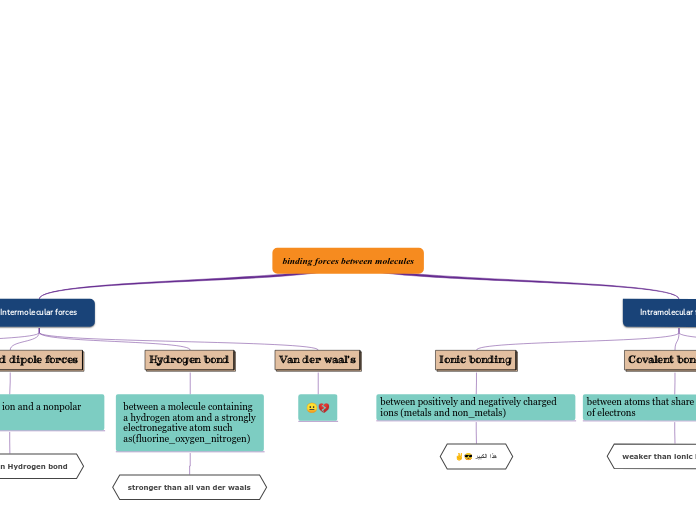
Covalent Bonds
Two (2) Non - Metals
Shared
Acids
Oxyacids
-ic endings
-ous endings
Nitric acid or Nitrous acid
Ionic Bonds
Ionic Compounds
Transferred
Binary Ionic
Polyatomic Ions
Charged Chemical
2 or more covalent bonds
Non - Metal Atoms
Non - Metal Ions
Negative Charge
H- = hydride
Negative Charge (Anion)
-ide ending
CH4
Suffix = hydride
Prefix = Tetra
H2O = dihydrogen oxide
Water (common name)
Metal Atoms
Metal Ions
Electric Charge (-)
Positive Charge (Cation)