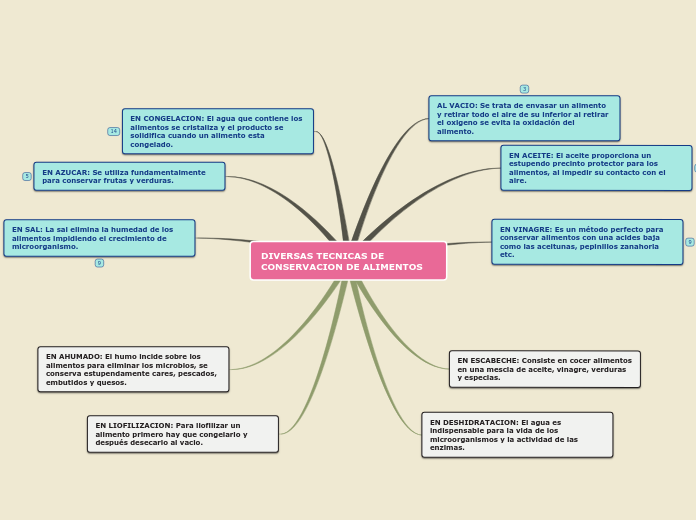
Inorganic Qualitative Analysis
basic radicals or( cations)
Group VI
They have no common reagent
Flame test:
a golden yellow
Sodium (Na+)
Potassium (K+)
Magnesium(Mg+)
Salt solution + Sodium hydroxide solution (NaOH):
Ammonium(NH4+)
Group V : Ammonium Carbonate Group
Salt Solution + NH4Cl solid + NH4OH solution + (NH4)2CO3
go to step 6
Gives white ppt,Flame test
green colour
Barium Ba2+
a crimson red
Strontium Sr2+
a brick red
Calcium Ca2+
Group Iv: Ammonium Sulphide Group
Group III: Ammonium Hydroxide Group
go to step 4
reddish brown gelatinous ppt
Iron(III), Ferric ,Fe+3
dirty green precipitate
Iron(II), Ferrous,Fe+2
grayish green
Chromium Cr+3
white gelatinous
Aluminum Al+3
Group II: Acid Sulphide Group
go step 3
***Sub Group II – B***Salt Solution +
dil . HC1 +
hydrogen Sulphide Solution (H2S )
yellow ppt
Tin ( IV )
Stannics Sn ++++
Tin ( II )
Stannous Sn ++
orang red ppt
Antimony Sb +++
***Sub-group II-A***Salt solution +dil. HCl + hydrogen Sulphide solution (H2S)
Salt solution + (solid) (NH4Cl) +
(NH4OH) + solution(H2S)
go to step 5
Gives a black ppt
Nickel Ni+2
Gives a black ppt.
Cobalt Co+2
Gives a buff ppt
Manganese Mn+2
Gives a white ppt
Zinc Zn+2
Mercury(II),Mercuric Hg++
a brown ppt
Bismuth Bi+++
black ppt
Copper Cu++
canary yellow
Cadmium Cd++
Group I: Dilute Hydrochloric Acid Group
go step 2
Salt solution + dil. Hydrochloric acid
** White ppt ** Salt solution + potassium chromate solution (K2CrO4))
brown ppt
Mercury (I)
Mercurous Hg22+
A yellow ppt.
Lead Pb++
brick red ppt
Silver Ag+
acid radicals or (anions)
3) Salt solution + BaCl2:
white precipitate
Salt solution +AgNO3
white ppt
sulphate
white ppt
which give brown ppt. after boiling
borate.
phosphate
2) Solid salt + conc. H2SO4:
(Go to step 3).
-ve
If a small piece of copper metal and drops of water are added, dense brown fumes
Tha anion is Nitrate
Violet fumes are evolved, and a brown or black
The anion is iodide.
Raddish fumes
The anion is Bromide.
colourless gas
The anion is chloride.
1) Solid salt + dil. HCl:
Observation
-ve
(Go to step 2).
paper black
The anion is sulphide
yellow precipitate
The anion is thiosulphate.
Colourless gas with Pungent odour
The anion is sulphite
Pungent brown fumes are evolved.
The anion is Nitrite
Effervescence and a colourless odouless gas is evolved.
The anion is carbonate or bicarbonate .
1 )Salt solution + magnesium sulphate solution
No ppt
bicarbonate
White precipitate
carbonate