arabera Andrew Swenson 17 years ago
278
31337 1-1/-\x0|2
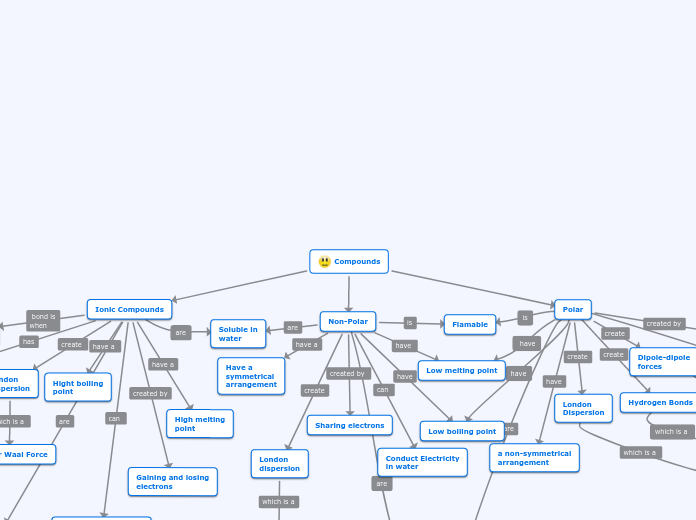
The text outlines the basic principles for naming various chemical compounds, focusing on hydrocarbons, acids, ionic compounds, and molecular compounds. For hydrocarbons, it explains the use of suffixes like -ane, -ene, and -yne, and notes that adding "