arabera khushi sharma 5 hours ago
6
Biochemistry
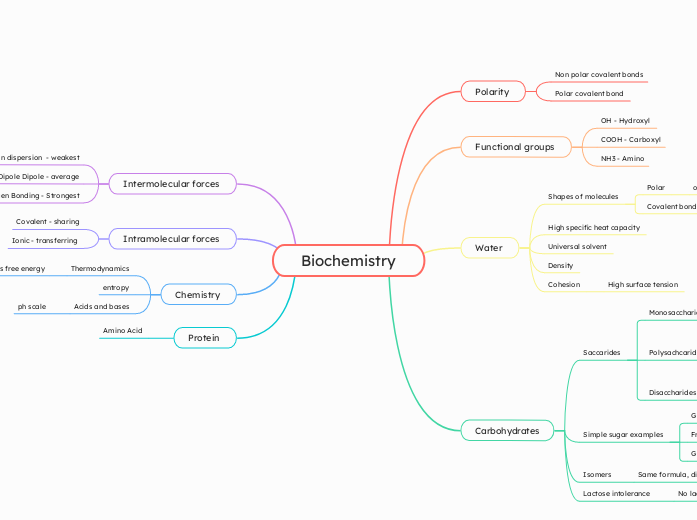
The study of biochemistry delves into the chemical processes within and related to living organisms. It covers various concepts such as intermolecular forces, including London dispersion, dipole-dipole interactions, and hydrogen bonding, with hydrogen bonds being the strongest.