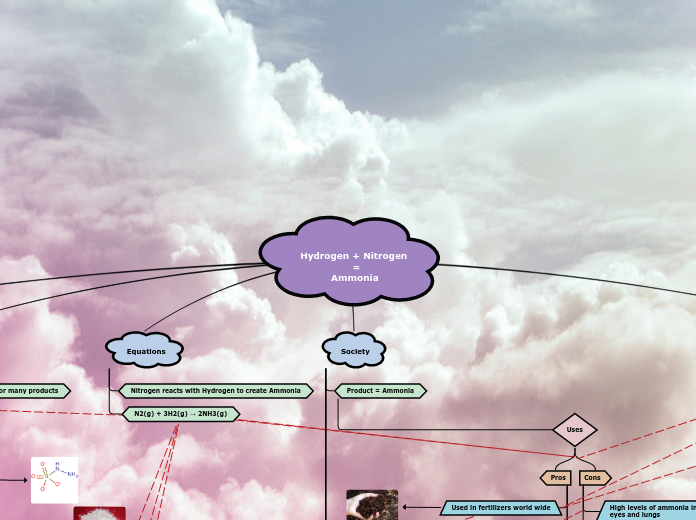
Hydrogen + Nitrogen
=
Ammonia
Environment
does not bioaccumulate
Ammonia isn't long lasting in nature
Ammonia is part of the nitrogen cycle
Ammonia is found in the environment in soil, air, and water.
Society
Regulations
Cost of production = about $350/t
Production = 3,300 tonnes per day
Health concerns
Product = Ammonia
Uses
Cons
High levels of ammonia in air is irritating to skin, eyes and lungs
Pros
Found in a lot of cleaning products
Can be formed into Ammonium salts and other types of Ammonium products
Used in fertilizers world wide
Equations
N2(g) + 3H2(g) → 2NH3(g)
Nitrogen reacts with Hydrogen to create Ammonia
Science
Properties
Ammonia
Corrosive
Toxic
PH level of 11
Pungent
Nitrogen
Non-corrosive
Gaseous
Hydrogen
chemical
Non-toxic
Non-metallic
Flamable
Physical
Tasteless
Odourless
Colourless
Energy
Requires catalyst
Elevated temperature
(400–550 °C )
Requires high pressure
(100–1,000 atmospheres)
Reactions
Hydroxylamine
Hydrazine
Subtopic
Ammonia is the base for many products
Ammonium
Ammonium sulphate
House hold products
Nutrients
Technology
The technology in making the catalyst to use in ammonia
Not a lot of technology are used to make Ammonia but Ammonia is used in technology to produce or create other things