Intermolecular Forces (IMF)
The forces of attraction between molecules
State of Matter
State of matter is another physical property that can be observed and/or measured like solubility that relates directly to strength of IMFs.
The stronger the IMFs, the higher the boiling and melting point will be.
(Textmap, 2020)
Partially hydrogenated oils are created because they are cheaper than animal fats and they can be modified to a desired consistency, such as that of butter as seen in margarine. Hydrogenation works by creating an alkane (saturated) from an alkene (unsaturated) through a hydrogenation reaction (Textmap, 2020). By the same principles above, (flat stacking due to only single bonds) the melting point increases and therefore these partially hydrogenated oils are able to be solid at room temperature due to increased IMFs.
An example of a higher melting point can be seen in lipids that are solid at room temperature, like butter. This is because butter is a saturated fat. Saturated fats contain only single bonds between carbons. This allows them to lay in a flat stack, thereby increasing the amount they touch which also increased IMFs due to proximity. In the case of unsaturated fats, such as olive oil, the IMFs are not as strong due to the double and triple bonds (and therefore not flat stack structure) and therefore liquid at room temperature.
(Wayne, 2018)
Simply put, the stronger the IMF the more energy needed to break the bonds, hence the higher the melting and boiling point of a substance will be
Solubility
"Like dissolves like"
Polar solutes dissolve better in polar solvents, and non-polar solutes dissolve better in non-polar solvents
Substances that are opposites cannot dissolve well due to the fact that molecules are unable to make strong intermolecular forces.
The strength of IMF and solubility has a direct relationship. Solubility works by molecules lining up opposite dipoles with strong enough forces that it causes solvation.
"The universal solvent" water
Although water is generally referred to as the universal solvent, this is not the case. While a great number of biological compounds dissolve in water, there are a number that do no not, for example combining oil and water.
While oil and compounds that contain mainly water do not normally mix, there is a process called "emulsification" that allows for this to happen.
While these compounds can use LDFs to bond without emulsification, these bonds are not strong and do not last long (hence a dressing needing to be shaken right before serving if it is not emulsified).
(Ahmed, 2020)
The general makeup of a surfactant molecule, with one end attracted to water, and the other repelled by water.
A surfactant contains both hydrophilic (water loving) and hydrophobic (water fearing) groups. Surfactants are added to a mixture where molecules would not usually mix to decrease surface tension and allows the hydrophilic ends of the surfactant molecule to line up with the water molecules (or polar) and the hydrophobic ends to line up with the oil (or non-polar). Examples of this include oil and vinegar salad dressings, and soap. Through the process of adding surfactants, emulsification occurs (Textmap, 2020).
There are certain molecules that contain distinctly non-polar bonds and are hydrophobic.(Textmap, 2020).
Water is an especially powerful solvent due to its polarity and the formation of hydrogen bonds, which are the strongest of the 3 IMFs discussed.
Water contains a positive partial charge (hydrogen) and a negative partial charge (oxygen). This allows it to bond with many different types of molecules. For an example, when looking at the solubility of NaCl in water, the slightly positive H is attracted to the negative Cl, whereas the slightly negative O is attracted to Na+.
(Khan Academy, n.d.)
Why are some alcohols able to dissolve in water and others are not?
Water can form hydrogen bonds with these smaller alcohols due to the fact H will bond to their hydroxyl groups. The energy gained is enough to make up for that which is lost through the alcohol bonds being broken up. When alcohol chains get longer (around 4-5 carbon length) there is larger hydrophobic (non-polar) areas and therefore becomes insoluble.
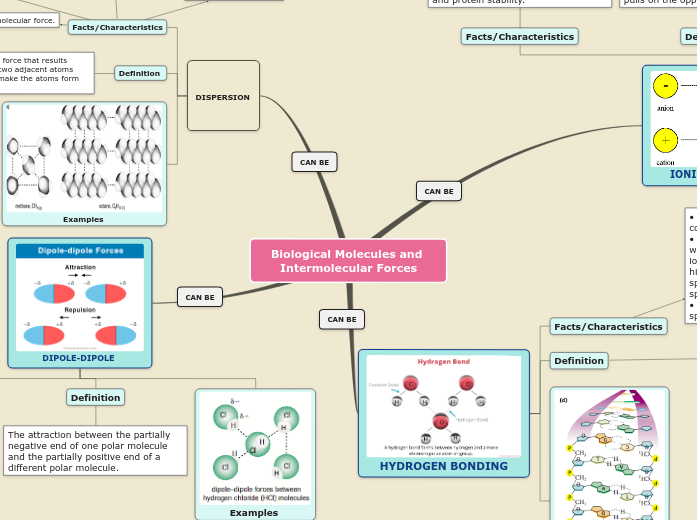
Types of IMFs
Hydrogen bonding
(Chemistry Learner, 2024)
Involves a "donor" and an "acceptor" molecule. The hydrogen atom used in the bond comes from the molecule referred to as the donor whereas the acceptor provides the electronegative atom which contains the lone pair of electrons.
Occurs between partially charged positive hydrogen and a very electronegative atom through the use of a polar covalent bond.
Attaches specifically to the lone pair of electrons of the electronegative atom
Examples of highly electronegative atoms include: nitrogen, oxygen and fluorine.
Strongest of the three IMFs discussed
Hydrogen bonding is a special case of dipole-dipole bonding.
Dipole forces
(Chemtalk, n.d.)
An example of this is HCl. The chlorine atom is more electronegative than the chlorine (thereby having a partial negative charge) whereas the hydrogen has a partial positive charge. You can see this occurring in the photo with the dotted line representing the IMF.
Happen between polar molecules with permanent dipoles (partial charges). These occur due to differences in electronegativity. The negative partial charges will line up with the positive charges of the neighbouring molecule resulting in a strong bond (Kofoed, Miller & Utah State University, 2023).
London dispersion forces (LDFs)
Also referred to as Van der Waals forces
(The OrganicChemistry Tutor, 2018)
Can be stronger or weaker depending on shape, ie two straight chain compounds will have more molecules as close neighbours, this results in stronger forces because of the close proximity.
In LDFs, a temporary dipole is created due to random movement of electrons. For example, a
temporary dipole can be created when
more electrons (negative charge) find themselves more concentrated on one side of the molecule. This will induce the molecule beside it to have an induced dipole so that negative and positive sides line up., therefore creating a momentary force that attracts the two molecules together, as seen in the photo.
Occurs between all molecules
Weakest of the three IMFs