Conductivity is never possible in solid states because the ions need to be in motion in order for electricity to be conductivity.
Want to test yourself on all of this information you've just learned? Click the small purple Q to the right---------------->
Test yourself with flashcards, quizes, and games!
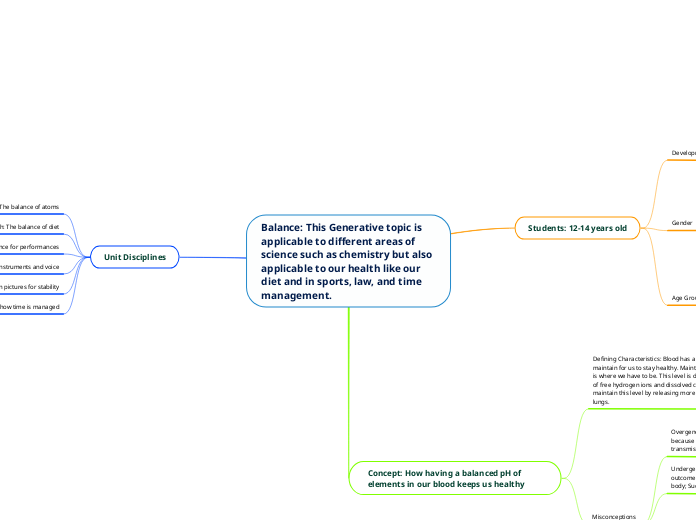
Hydrogen bonding Hydrogen bonding is a force of attraction occurring between a hydrogen atom and an atom of a higher electronegativity, making the bond highly polar covalent. The hydrogen atom bonds exclusively to the one of the following atoms: nitrogen (N), oxygen (O), or fluorine (F). These hydrogen bonds are quite strong, culminating in the need for high melting and boiling points in order to break them.
**Remember: in hydrogen bonding (or H-bonding for short) , the hydrogen will only bond with either N, O, or F. ***
When interacting with other water molecules, the hydrogen atom, which is partially positively charged, will be attracted to the partially negatively charged oxygen of another atom. Due to water's bent shape, the molecules are going be very spread out when frozen. This crystal lattice that forms thus has a lower density than liquid water, which is why ice floats in water!
Click the small Google Drive icon to the left to for futher clairification on the structure of ice
----------------------------------------------------->
Dipole-dipole Dipole-dipole forces are the result from the attraction of the partial charge from one molecule to a molecule with the opposite partial charge. These forces are the consequence of interaction between the positive and negative end of two polar molecules. That means that these are interactions between two permanent dipoles, as opposed to London Dispersion Forces, which are caused by The partial positive end attracts the partial negative end (and vice versa, of course). These dipole-dipole interactions create attraction between molecules.
LONDON DISPERSION FORCES
Yes! It does... but so do all other atoms. It has London Dispersion Forces, a variant of van Der Waals forces. Every single atom has LDF's. These forces are very weak and occur in all atoms because, though charges in non-polar compounds are distributed equally, unprompted dipoles still occur because of the fact that electrons are always in motion. The spontaneous dipoles that occur make for the atom to have momentary polarity, meaning that the atom has a short-term polar and negative end. This causes attraction between the (temporary) negative end of one atom with the (temporary) positive end of a neighbouring atom. This resulting attraction is what is called a London Dispersion Force (or LDF for short). Remember that LDF's are caused by temporary dipoles, not the other way around! The reason for London Dispersion Forces being so weak is that the induced dipole is only temporary. **LDF's are also sometimes called induced dipoles***
Click the small Google Drive icon to the right to see a diagram of London Dispersion Forces------------------------------------------>
Intermolecular + Intramolecular
Non-polar covalent bond
This is a type of bond where the electrons are shared equally between atoms in a compound.
NON-POLAR COVALENT MOLECULE
Yes...if the bonds within the molecule are polar, the molecule itself is also going to be non-polar. This is not always the case, however, for molecules with polar bonds. Refer to the polar covalent bonds strand for more information on this.
Electronegativity (or EN for short) is
the measure of an atoms ability to attract bonding electrons to themselves. EN is calculated by subtracting the smaller electronegativity from the larger one. Electronegativity values can generally be found underneath the element name on the periodic table or underneath the element number. Click the small youtube icon to the right.
... the forces present in the molecule are intermolecular. Intermolecular means "in between" molecules, but you could think of it as "inter molecules" to help you remember.
The forces are intramolecular. These forces hold a particle together and are, for this reason, stronger than intermolecular forces. They also account for the chemical properties observed. Though, there are different categories...
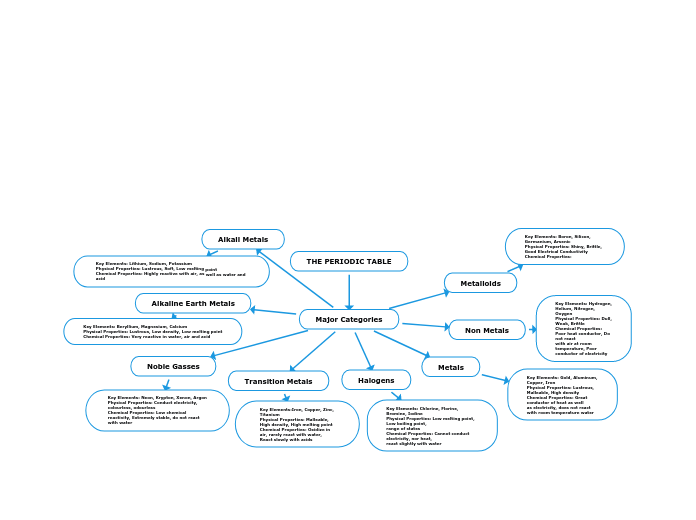
Covalent bonds
Covalent bonds happen when atoms are shared between two atoms, in order to become stable. Covalent bonds have low melting points (since the bonds are relatively weaker); are soft, waxy and brittle; and, in general, don't conduct electricity.
Click on the small Youtube icon to the right to find out how ------------------------------->
Polar covalent bond
This is a type of bond where the electrons are shared unequally between atoms in a compound. As a result, the compound has a positive and negative end, hence the use of the word "polar". As I said, covalent bonds don't usually conduct electricity. With polar covalent bond, however, conductivity is possible in a liquid state if there is a hydrogen in the compound and it's acting as a cation.
Not necessarily...
Recall that in order for a molecule to be polar, it must have a positive and negative end. This means that the molecule is asymmetrical due to the unequal distribution of electrons, resulting in the polar ends. But let's take methane, CH4 (4 is a subscript), as an example. We know that CH4 is a covalent bond as both carbon (C) and hydrogen (H) are nonmetals. If we calculate the electronegativity, each bond should be polar. Refer to the diagram for further clarification. You see how the shape of CH4 is symmetrical? This means that each of the polarities in the C-H bonds cancel each other out, making that the overall molecule doesn't have any positive and negative end.
Then the molecule is polar, because it has a positive and negative end.
Ionic Bonds
The bonding within the molecule is an ionic bond. Ionic bonding happens when electrons are transferred from one atom to the other in a compound, in order for each atom to become stable. Ionic compounds are able to conduct electricity because the are made up of ions. Ionic compounds have high melting and boiling points (because they are very strong intramolecular forces), are hard and brittle at SATP, and conduct electricity in liquid form.
Click the small Google Drive icon attached to this, on the left ------->
Intermolecular + Intramolecular Forces
An enduring attraction between a group of atoms resulting in the formation of compounds.