arabera Aryan Singh 3 years ago
292
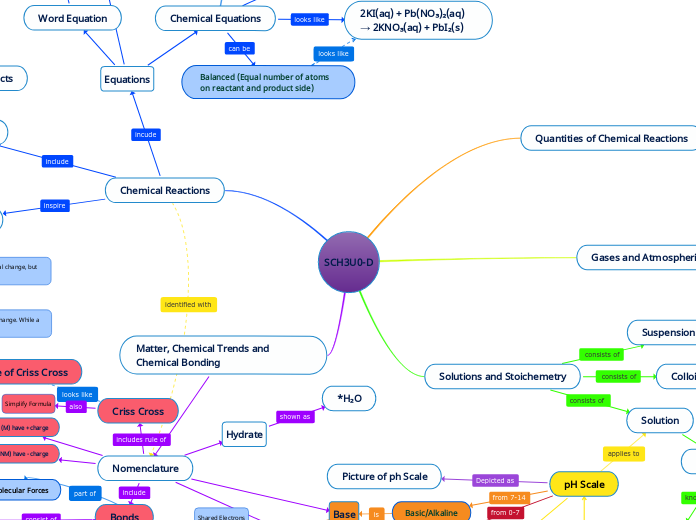
SCH3U0-D
arabera Aryan Singh 3 years ago
292

Honelako gehiago
δ+
δ-
Atoms
Parts
Shells
Electrons (- charge)
Valence Electrons
Stability
Octet Rule
Boron and Beryllium
Nucleus
Neutrons (no charge)
Protons (+ charge)
Properties
Atomic Mass
Mass Number
Isotopes
Atomic Number
Anion
Cation
Ionic
Ionic Compound
Multivalent Compound
Metal has more than one charge
roman numerals (e.g. I, II, IV, V)
Polyatomic Ion
Covalent
Diatomic
HOFBRNCl
Molecular Compound
Metallic
M + M
*H₂O
Binary Acid
Oxyacid
Simplify Formula
Net Ionic Equations
Stoichiometry
2KI(aq) + Pb(NO₃)₂(aq) → 2KNO₃(aq) + PbI₂(s)
Balanced (Equal number of atoms on reactant and product side)
Sodium + Chloride -> Sodium Chloride
Non-metal Oxide
Metal Oxide
Activity Series
Element's reactivity relative to each other
Displacment occurs quicker
Compound's element if it's below
Single Displacement
A(non-metal) + B(metal)C -> AB + C
A(metal) + B(metal)C -> AC + B
Double Displacement
AB + CD -> AD + CB
Precipitate
ionic formed
insouluble (connect to solutions unit)
Fuel
Incomplete Combustion
limited oxygen
Carbon Dioxide, Water Vapor, and Energy + Carbon Monoxide and/or Soot (Carbon)
Complete Combustion
extra oxygen gas
"Connect the extra and limited to the concept of excess reagent and limited reagent"
Carbon Dioxide, Water Vapor, and Energy
A salt and water
AB -> A + B
A + B -> AB
Painting a car (change in color) is a physical change. While a solution changing color is a chemical change
Chemical Composition
Texture
Change in States of Matter
endothermic
exothermic
Melting Point
Boiling Point
Shape
Irreversible
Odor
Color
Bubbles/Gas
New Substance
Heat/Light
Solubility
Amount of solute per amount of solvent: e.g. 36.0 NaCl/100g of H20 at 20°C
Solubility Curves
Saturated
Supersaturated
Unsaturated
Below the line, any dissolved solute
Above the line, any dissolved solute
On the line, any dissolved solute
Solubility Factors
Subtopic
Pressure
Temperature
Concentration
C₁V₁ = C₂V₂
Molar Concentrations
M = n/V
Very Small Concentrations
Parts Per Trillion
ppt = solute/solution * 10^12
Parts Per Billion
ppb = solute/solution * 10^9
Parts Per Million
ppm = solute/solution * 10^6
Solution Concentrations
Percentage Mass(g) (%m/m)
Percentage Mass(g) by Volume(L) (%m/V)
Percentage by Volume(L) (%V/V)
% C (m/V) = msolute/Vsolution
Solute
Solvent
Golden Rule "Like Dissolves Like"