arabera Sahil Patel 4 years ago
2082
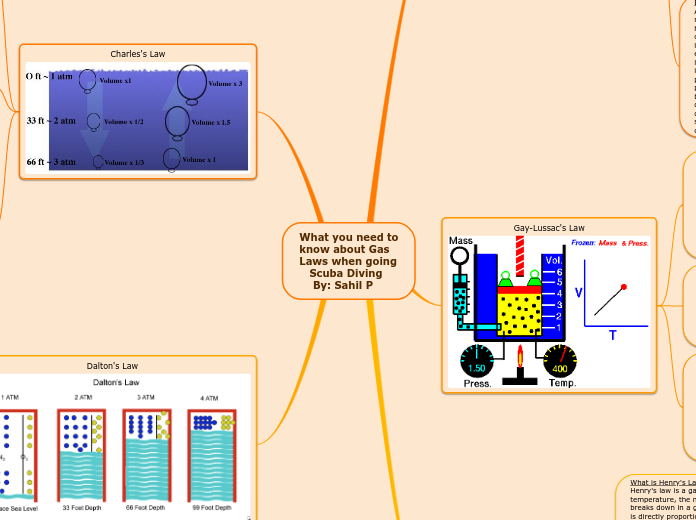
What you need to know about Gas Laws when going Scuba Diving By: Sahil P
arabera Sahil Patel 4 years ago
2082

Honelako gehiago