jonka Qabas Al-Jobori 1 vuosi sitten
88
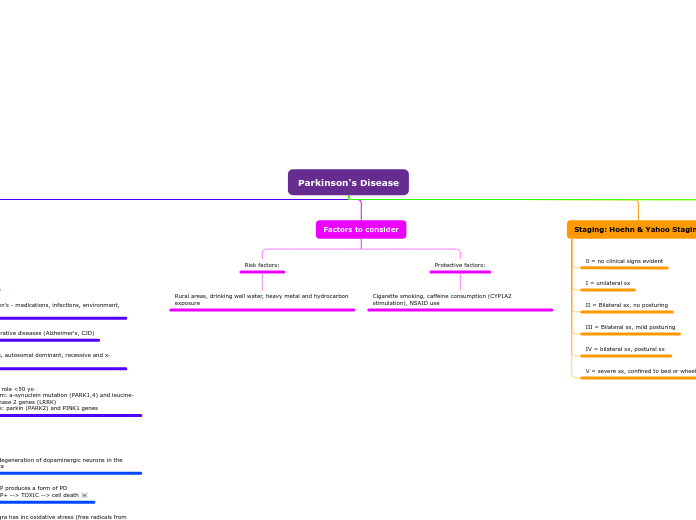
Parkinson's Disease
Parkinson’s disease is characterized by the degeneration of dopaminergic neurons in the substantia nigra, leading to a decrease in dopamine levels and resulting in motor impairments.
jonka Qabas Al-Jobori 1 vuosi sitten
88

Lisää tämän kaltaisia
What agent is FDA approved for the txt of PD psychosis? Pimavanserin
Agents: Ergotamine (bromocriptine, old, not used, pulmonary fibrosis) Nonergotamine (pramipexole/ropinirole) -reduce levo exposure, delay need to start levo tx -used as initial tx, in young adult pts or adjunct in older -Ropini: CYP1A2, used for RLS (restless leg syndrome), causes pts to fall asleep suddenly -Prami: sudden falling asleep, renal elimination -AEs: n, confusion, sedation, edema, vivid dreams -Uses: -younger pts (<65): for motor fluctuations -older (>65): hallucinations/ortho HypoTN
Can COMT inhibitors and dopamine agonists be used in combination to treat PD? Yes
Agents: Entacopone, Tolcapone -combo w/ carb/levo -also indicated for "off" eps -Tol causes liver problems, monitor LFTs -Delayed diarrhea, brownish-orange urine discoloration
Agents: Selegiline & Rasagiline -used early in tx, neuroprotective -sele: metab by CYP2B6/2C19 to L-methamphetamine. ODT bypasses GI and dec formation of the agent -AE's: N, dec appetite, ortho HypoTN, hallucinations, dyskinesias, insomnia -Rasa: metab by CYP1A2 to inactive metabolite. Ads like placebo.
Istradefylline: -Adenosine A2 receptor antagonist -adj w/ levo/carb for "off" eps -max dose for smokers >20 cig/d -DDI: metabolized by CYP1A1/CYP3A4
Safinamide: -selective, reversible MAO-B inhibitor -approved for adj txt w/ levo/carb for "off" eps -may also improve motor function
What does carbidopa do in the formulation with levodopa?? Prevent the GI effects of DOPA decarboxylase to allow increase L-Dopa absorption
What are the 4 motor complications assoc. w/ levo/carb therapy? End of dose wearing "off", delayed "on", freezing, and dyskinesias
Dyskinesias: L-dopa "on" periods -assoc. w/ peak DA lvls -lower drug dose = balance with AE vs. symptoms -add amantadine "off-period" dystonia: muscle contractions -feet, early morning hours, improvs w/ first drug dose -HS use of CR products L-Dopa or DA agonist -Baclofen and botox (??)
Delayed On: due to delayed gastric emptying or reduced absorption -chewing or crushing tabs/ODT on empty stomach -apopmorphine SQ, possible drug holidays Freezing: feeling like stuck to the floor -physiotherapy or walking devices -increase drug dose, MAO B inhibitor or DA agonist
Issues to be aware of. End of Dose Wearing Off AKA On/Off: occurs prior to next dose -happens due to inc loss of storage for DA and short L-DOPA 1/2 life -peripheral PK effects >> CNS effects -Give more frequently, add COMT or MAO B inhibitor. CR formulation can be useful -consider DA agonist during day or HS use of PO products
What agent can be initially used in PD pts that have symptoms WITHOUT significant impairment? Rasagiline
What is stage IV of PD according to the Hoehn and Yahr staging of severity scale? Bilateral symptoms and postural instability
L-AAD can be blocked by drugs like carbidopa that do not pass the BBB, thereby increasing L-DOPA lvls from meds and reducing AEs. L-DOPA is also metabolized by L-AAD.
NTs involved: -acetylcholine -GABA -Glutamate -enkephalins -Sub.P -adenosine -serotonin
-Reduced DA lvls -Inc ACh lvls (DA inhibits ACh, when it dec then ACh is inc, leading to tremors) -DA cells cont. to be destroyed and DA production declines -Progressive disease, sxs+severity will worsen
Symptoms: -decreased dexterity -dysarthria (difficulty speaking) -dysphagia (diff swallowing) -flexed posture -freezing at movement initiation -bladder problems Autonomic symptoms: -bladder problems Mental status changes: -dementia at later stages