Topic flotante
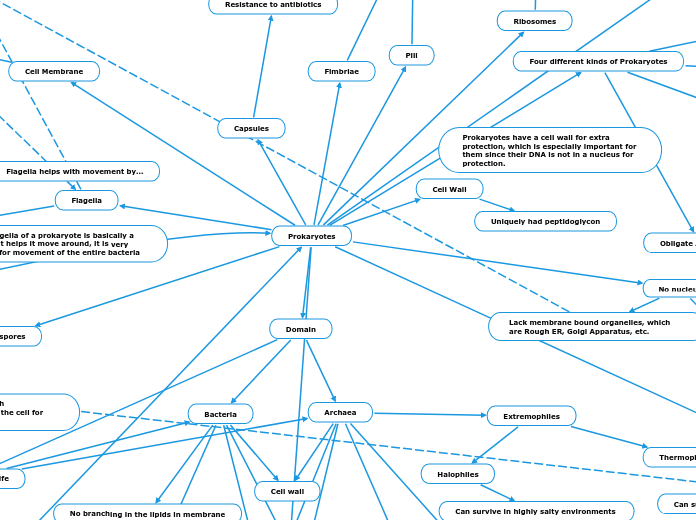
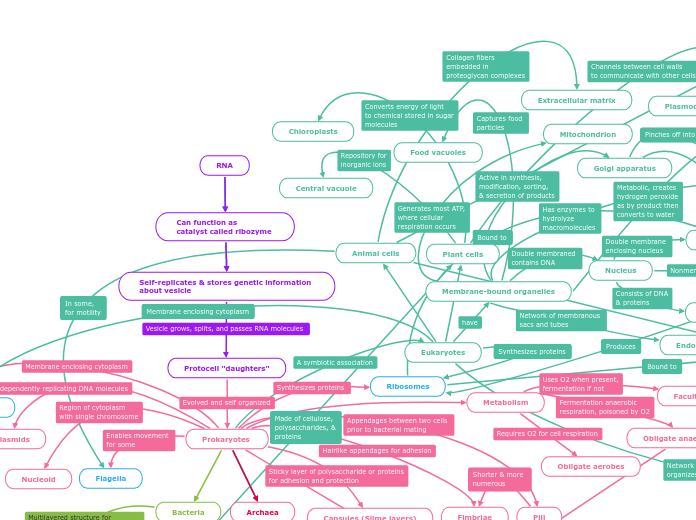
Prokaryotes have a cell wall for extra protection, which is especially important for them since their DNA is not in a nucleus for protection.
All of their genetic material is organized into chromosomes
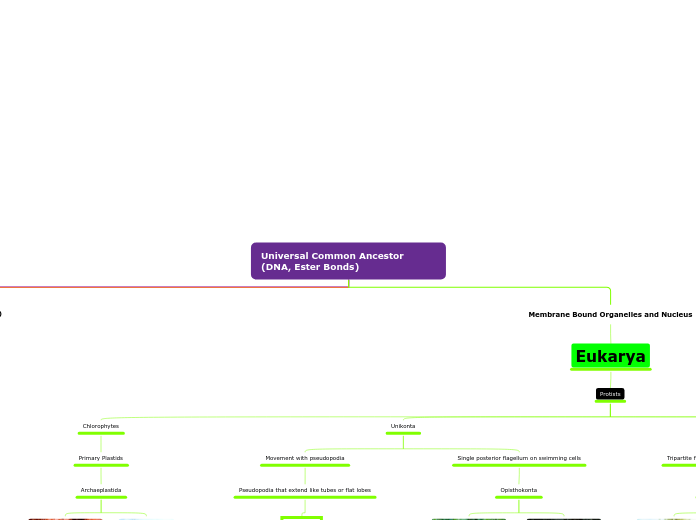
The Four kinds of Eukarya
Autotrophs are organisms that can make nutritional organic substances from inorganic molecules
There are two different types of prokaryotes, bacteria and archaea and they have a lot of similarities and differences. the main being that bacteria has a presence of peptidoglycan in the cell wall
Extremophiles are prokaryotes that can survive in extreme environments, there are a couple different types of extremophiles, depending on what that cell thrives in, but they are always extreme.
Fimbriae and pili are the prokaryotic sex parts of the cell, they connect to another cell and have prokaryotic sex, leading to the creation of more cells.
The flagella of a prokaryote is basically a tail that helps it move around, it is very useful for movement of the entire bacteria
A plasma membrane is basically a lipid bilayer where two rows of lipids come together where the hydrophobic tails are inside with each other and the hydrophilic heads are outside facing the cytoplasm and polar outside.
The DNA for prokaryotes is stored in a nucleoid instead of a nucleus. It is not as protected since there no nucleus. Which goes in part with how prokaryotes were the first cell to exist, eukaryotes were an evolution where it learned to protect its chromosomes and DNA
Obligatory means that the bacteria needs something to survive, if it is a facultative then it can survive with or without a certain thing. Anaerobe means that it has to do with oxygens, either needing it or not.
These are different inorganic molecules to show as examples
Amphipathic
Phospholipids form closed bilayers in water
Phospholipids
Phospholipids: made up of a glycerol linked to 2 fatty acids (not 3)
- It contains a phosphate group on the head
- The phosphate group is polar
Phospholipid Tail
Hydrophobic
Hates water : (
Phospholipid Head
Hydrophilic
Hearts water <3
Steroids
Steroids are very different from fats in the way that they contain 4 fused rings
HDL
High density lipoprotein or "good cholesterol"
Found in animal and a common components of membranes
LDL
Low density lipoprotein or "bad cholesterol"
Also a precursor of other steroids including sex hormones
Fats
Fats: made up of a glycerol + 3 fatty acids
-Main function: energy storage
-Ester linkages are what connect each fatty acid to an OH in glycerol
Fatty Acids
Unsaturated
Unsaturated fats come from plant sources and are liquid at room temperature. These fats have one or more double covalent bonds that are found within the carbon chain. These molecules do NOT have hydrogen atoms at every position along the carbon chain.
Monounsaturated Fats: one double bond
Polyunsaturated Fats: more than one double bond
Unsaturated Fatty Acids Isomers
Trans Isomers ( opposite side) AKA Transfats: the presence of a double bond with hydrogens on opposite sides.
Cis Isomers (same side): the presence of a double bond in cis causes the molecule to have a kink compared to the trans fatty acid.
Saturated
Saturated fats are solid at room temperature. There are no double covalent bonds between carbons because these molecules are saturated with hydrogen atoms at every position. They are also associated with an increase in cardiovascular disease.
Flagella helps with movement by...
Water (H2O)
Water is made from a covalent bond of 2 hydrogens and 1 oxygen. Oxygen is much more electronegative than hydrogen, thus creating a strong dipole.
This means that water can hydrogen bond and is one of the most polar molecules. This means that water has a ton of awesome properties.
Hydrophobic/hydrophilic interactions
Water-loving molecules exclude hydrophobic molecules such as lipids, which means that hydrophobic molecules stick together when in an aqueous environment. Amphiphilic molecules take advantage of the environment to surround hydrophobic molecules yet still flow with the surrounding water.
Hydrolysis/dehydration
Water is extremely vital when it comes to forming and breaking ester bonds. These are seen in Lipids, carbohydrates, proteins, DNA, and basically everything.
Strong intermolecular bonds
Water has strong intermolecular bonds as a result of bring so polar. This allows for a high heat of vaporization and high specific heat. In turn, this helps to regulate temperatures so biology can exist the way it does.
Universal Solvent
Water is the universal solvent because of its strong polarity and its relatively low molecular weight. Liquid water is everywhere.
Cellular Structure and Functions
Serve as fuel and building material
Chemical Bonds
Intermolecular Bonds
Polar molecules
Ion-dipole (4)
The strongest type of intermolecular bond. Seen commonly in dissolved solutions, such as salt water. Thus, salt water is more difficult to freeze and boil. This bond is between a fully charged ion and a partially charged side of a molecule. For instance, the negative Cl ion with the positive H´s in salt water.
Seen in the tiertiary structure of protiens.
Dipole-dipole (2)
Covalently bonded molecules that are polar have a dipole movement. The partially negative side of one molecule will bond with the partially positive side of another molecule. Stronger than london dispersion, but weaker than Ion-dipole.
Hydrogen bond (3)
A type of dipole-dipole where a hydrogen is covalently bonded to a N, O, or F. This hydrogen is then intermolecularly bonded to any polar N, O, or F element of another molecule
Hydrogen bonds are incredibly important. They hold together proteins, DNA, enable water to be water, create functional groups, and regulate temperatures. They are a stronger form of dipole-dipole
London Dispersion (1)
Every molecule has a set number of electrons. These electrons move randomly in an electron cloud. An instantaneous dipole movement occurs when electrons gather on one side of the molecule or atom. These instant dipole movements allow for instantaneous bonds. This bond is ALWAYS present but is the weakest out of the bonds listed. Most prominent between nonpolar molecules. Increases with surface area and amount of electrons in a molecule.
Nonpolar
Intramolecular
Ionic
Between two fully charged ions. Giving and receiving electrons. Stronger as the charges are full.
Covalent
Between two atoms. Electrons are shared bwteen atoms. Atoms with a higher electronegativity will pull electrons more therefore creating a polar molecule. When a molecule shares electrons equally, it is known as a nonpolar molecule.
Covalent bonds are seen in biology as glycosidic, peptide, ester, phosphodiester, disulfide bridge bonds.
Cell
They were the first cell ever created, where we started to get everything from
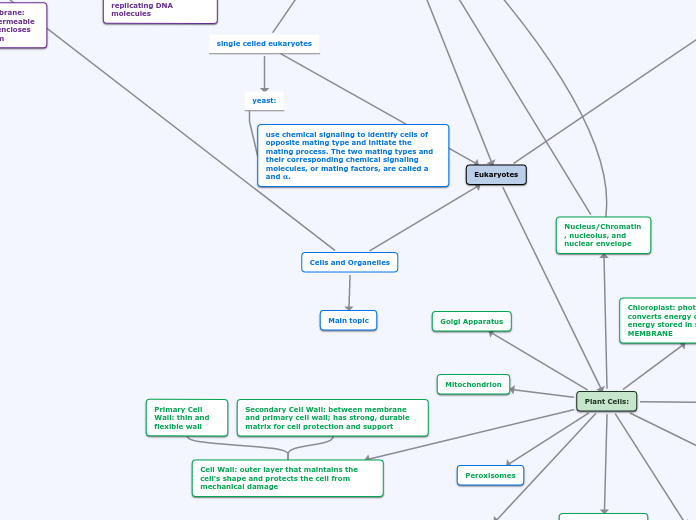
Eukaryotes
cell junction
gap junction
desmosomes
tight junction
cilia
endomembrane system
Animal Cell
ribosome
site of protein synthesis
golgi apparatus
synthesis, modification, sorting, secretion
mitochondria
intermembrane space, outer membrane, inner membrane
cellular respiration and ATP
peroxisome
metabolic function
lysosome
storage disorder
inherited metabolic disorder and buildup of toxic materials in cells
autophagy
fuses with vesicle containing damaged organelles, which hydrolytic enzymes digest
phagocytosis
contains active hydrolytic enzymes, which digest food particles
digestive organelle
microvilli
increase cell surface area
cytoskeleton
microtubules
organelle movements
chromosome movements in cell division
cell motility
maintenance of chell shape
intermediate filaments
anchorage of nucleus + other organelles
formation of nuclear lamina
microfilaments
cytoplasmic streaming
changes in cell shape
cellular contraction
Centrosome
where microtubules are initiated
Endoplasmic Reticulum
Smooth ER
doesn't contain ribosomes
Rough ER
contains ribosomes
Plant Cell
cell wall
maintains cell's shapes and protects cell from damage
plasmodesmata
cytoplasmic channelsd that connect cytoplasms
chloroplast
photosynthesis, converts sunlight into chemical energy
vacuole
central vacuole
repository for inorganic ions
contractile vacuole
pump excess water out
food vacuole
when cells engulf food
storage, breakdown waste, hydrolysis
cytoplasm
Nucleus
progeria
a childhood disorder caused by point mutation. cells could die prematurely
nuclear membrane
chromatin
proteins
DNA
nucleolus
nonmembranous structure producing ribosomes
nuclear envelope
double membrane enclosing nucleus
Prokaryotes
Four different kinds of Prokaryotes
Facultative Anaerobes
Can survive without O2 by fermentation
Can survive with O2
Obligate Anaerobes
Energy source is fermentation
The chemical breakdown of a substance by bacteria, yeasts, or other microorganisms. They get their energy by breaking down something else, which in this case is oxygen gas. It needs to break down oxygen gas to feed itself, that's the only way it knows how to survive or CAN survive.
Cannot survive with O2, needs to not have it, O2 is toxic to it.
Obligate Aerobes
NEEDS O2 to survive
Heterotroph
Chemoheterotroph
Needs organic compounds to survive
Photoheterotroph
Needs light to survive
Endospores
Helps the cell to survive harsh environments and can live in the cell for years
Autotroph
Chemotroph
Needs inorganic molecules to survive
Makes organic compounds out of inorganic molecules
C,H,O,N
Stanley-Millers Hypothesis/experiment
Based on the hypothesis that early life elements were being spouted from underwater volcanoes
The elements then became chemical compounds through chemical evolution
The compounds were put into a boiling water chamber to express the hot waters due to the volcanoes in early earth.
The that vapor was put into another chamber with electricity in it, symbolizing naturally occurring electricity through lightning
Organic compounds were the result of the experiment
Etc
Fe2+
NH3
Phototroph
Needs LIGHT to survive
They are both energy sources for an autotroph, just in different ways depending on what the environment is
Ribosomes
Synthesis of protein
Capsules
Resistance to antibiotics
Polysaccharide layer that lies outside of the cell envelope
Domain
3 main domains of life
Eukarya
Anamalia
Plantaea
Fungi
Protists
Does have a nucleus where the DNA are protected, in comparison to Bacteria and Archaea, where neither of them have a nucleus
Archaea
Methanogens
They are strict obligate anaerobes
Have developed themselves to be able to survive in methane (usually in swamps)
Extremophiles
Thermophiles
Can survive in extreme temperatures
Halophiles
Can survive in highly salty environments
Presence of branched lipids in membrane
Bacteria
Does not have membrane enclosed organelles
Presence of peptidoglycan in cell wall
Presence of a cell wall
Cell wall
No branching in the lipids in membrane
Pili
Prokaryotic sex
Interact with other bacterial cells and are able to move strain of things around
Chromosome (DNA)
Nucleoid
Cell Membrane
Plasma membrane
Lipids bilayer that surrounds the cytoplasm
Lipids have ester bonds that are created and join things together by dehydration reactions
No internal membrane bound organelles, whivh is what differs the most boldly of prokaryotes and eukaryotes, along with the absence of a nucleus
Fimbriae
Flagella
Movement
Cell Wall
Uniquely had peptidoglycon
No nucleus
Lack membrane bound organelles, which are Rough ER, Golgi Apparatus, etc.
Biomolecules
Lipids
Carbohydrates
Serve as fuel and building material
Sugars + Polymers of Sugars
Monosaccharides
Disaccharides
Formed when a dehydration reaction joins two monosaccharides
Made up of C, H, OH and CO groups
Ketoses
When CO group is in the middle of the chain there are called
Aldoses
When the CO group is at the end of the chain the sugars
Simplest Sugars
In aqueous solutions they form rings
Glucose
Alpha Glucose
Digestable bc OH group is at the bottom
Beta Glucose
Undigestible because the OH group is at the top of the ring
Polysaccharides
Storage Polysaccharides
Glycogen
Starch
Amylose
No Branching
Differ in structure
Amylopectin
Some branching
Plants
Structure Polysaccharides
Cellulose
Made up of Beta glucose
Parallel chains held together through hydrogen bonds and form microfibrils.
PROTEIN AND DNA
DNA STRUCTURE
DNA is made of two linked strands that wind around each other to resemble a twisted ladder, a shape known as a double helix.
DNA FUNCTION
DNA contains the instructions needed for an organism to develop, survive and reproduce.
DNA sequences must be converted into messages that can be used to produce proteins
DNA cannot function
without protein
and vise Versa
transmit signals to coordinate biological processes between different cells, tissues, and organs
assist with the formation of new molecules by reading the genetic information stored in DNA.
proteins provide structure and support for cells.
Immunological
DNA also contribute to the pathogenesis of autoinflammatory diseases and cancer.
Genetics
chromosomes are made up of thousands of shorter segments of DNA, called genes.
These instructions are stored inside each of your cells, distributed among 46 long structures called chromosomes.
DNA holds genetic information that determines an organisms traits
Structural
dependent on the sugar phosphate backbone and the bases.
Deoxyribonucleicacid
DNA REAL TERM
forms the structural framework of nucleic acids, including DNA and RNA.
Each strand has a backbone made of alternating sugar and phosphate groups. Attached to each sugar is one of four bases adenine (A), cytosine (C), guanine (G) or thymine (T).
Adenine--->thymine
Cytosine--->Guanine
Sugar Phosphate Backbone
joins together nucleotides in a DNA sequence
Each Sugar attaches to a base
part of DNA that stores information
each base contains nitrogen
Each base is held together
through hydrogen bonds
PROTEIN STRUCTURE
The sequence of amino acids linked together to form a polypeptide chain. Each amino acid is linked to the next amino acid through peptide bonds created during the protein biosynthesis process.
The primary structure of protein
forms an amino acid chain.
The amino acids are stabilized by
Hydrogen bonds
PROTEIN FUNCTION
cell shape and inner organization
akin to a skeleton, and they compose structural elements in connective tissues like cartilage and bone in vertebrates
Ribosomes are responsible for synthesizing
proteins and rna
product manufacture and waste cleanup
breaks down proteins, the building blocks and mini-machines that make up many cell parts.
receive signals from outside the cell and mobilize intracellular response
Signals most often move through the cell by passing from protein to protein, each protein modifying the next in some way
signaling pathway
These hydrogen bonds
create alpha helix and beta pleated
sheets for the secondary structure
amino acids are linked together by peptide bonds, thereby forming a long chain
Hydrogen bonds between sections of the protein chain are responsible for the secondary structure of the protein
The tertiary structure of a protein refers to the overall three-dimensional arrangement of its polypeptide chain in space.
Three dimensional arrangement of
its polypeptide chain
The quaternary structure of a protein is the association of several protein chains or subunits into a closely packed arrangement.
the association of several protein chains or subunits into a closely packed arrangement