Chemistry review
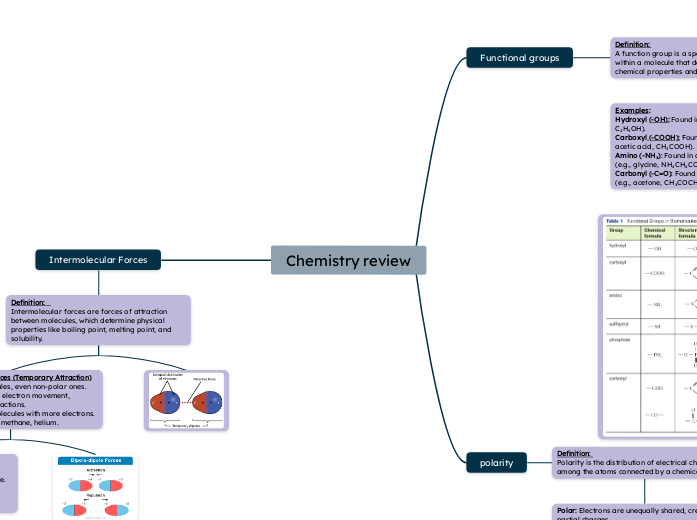
Intermolecular Forces
Definition: Intermolecular forces are forces of attraction between molecules, which determine physical properties like boiling point, melting point, and solubility.
London Dispersion Forces (Temporary Attraction)
-Happens in all molecules, even non-polar ones.
-Caused by temporary electron movement, creating tiny weak attractions.
-Stronger in bigger molecules with more electrons.
Example: Oxygen gas, methane, helium.
Dipole-Dipole Forces (Opposites Attract)
-Happens in polar molecules, where one side is slightly positive and the other is slightly negative.
-The positive side of one molecule attracts the negative side of another.
Example: Hydrogen chloride, methyl chloride.
Hydrogen Bonding – Strong Dipole-Dipole
-Special dipole-dipole force when hydrogen is -bonded to nitrogen, oxygen, or fluorine.
-Much stronger than normal dipole-dipole forces.
-Makes water stick together, which is why it has a high boiling point.
-Example: Water, ammonia, DNA.
polarity
Definition: Polarity is the distribution of electrical charge among the atoms connected by a chemical bond
Polar: Electrons are unequally shared, creating partial charges Non-Polar: Electrons are equally shared between atoms Ionic: Electrons are fully transferred from one atom to another
Functional groups
Definition: A function group is a specific group of atoms within a molecule that determines the molecule's chemical properties and reactions
determines
Examples:
Hydroxyl (-OH): Found in alcohols (e.g., ethanol, C₂H₅OH).
Carboxyl (-COOH): Found in carboxylic acids (e.g., acetic acid, CH₃COOH).
Amino (-NH₂): Found in amines and amino acids (e.g., glycine, NH₂CH₂COOH).
Carbonyl (-C=O): Found in ketones and aldehydes (e.g., acetone, CH₃COCH₃)