types include
Solubility Curve
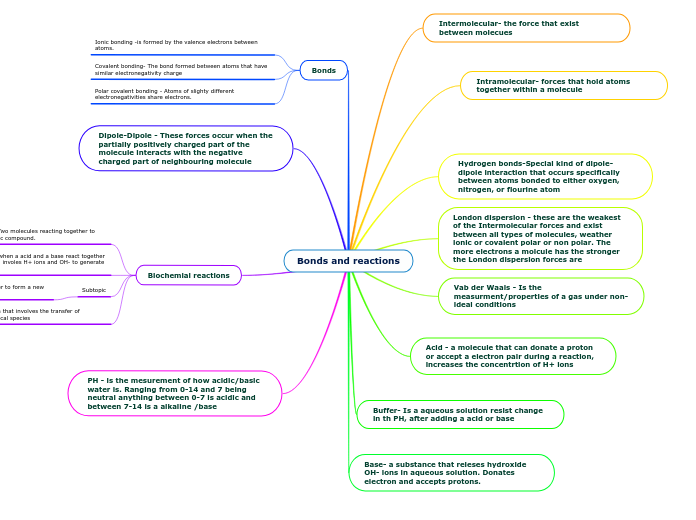
INTERMOLECULAR FORCES
Hydrogen bonding -a type of dipole-dipole however it happen between hydrogen and either oxygen, nitrogen or flourine
Dipole- dipole occurs between one positive end and one negative end.
London dispersion : Electrons are constantly moving. Bunching up causing one negative and the other positive result in a temporary attraction. It will happen with polar and non-polar
can be calculated of
Molar mass
Molecules
Mass and mol
grams
Mass
Determine whether molecule is
VSPER
non-polar
ionic bond
polar
Attach photo of my molarity equation
0 < ∆EN ≤ 1.7
∆EN >1.7
∆EN = 0
negative
Anions
postive
cations
Geometry
electron pairs
lone pairs
Reactivity of Non Metals
Bohr’s Model
Thompson Model
Rutherford’s Model
Reactivity of Metals
Electronegativity
Electron Affinity
First Ionization Energy
Atom diagram
Chadwick model
Atomic Radius
atomic number
mass number
charge
Radio Isotopes
Isotopes
neturons
Atoms
the ratio of the amount of solute to the
amount of solution
concentrated
Dilution
FACTOR LABEL METHOD
Units
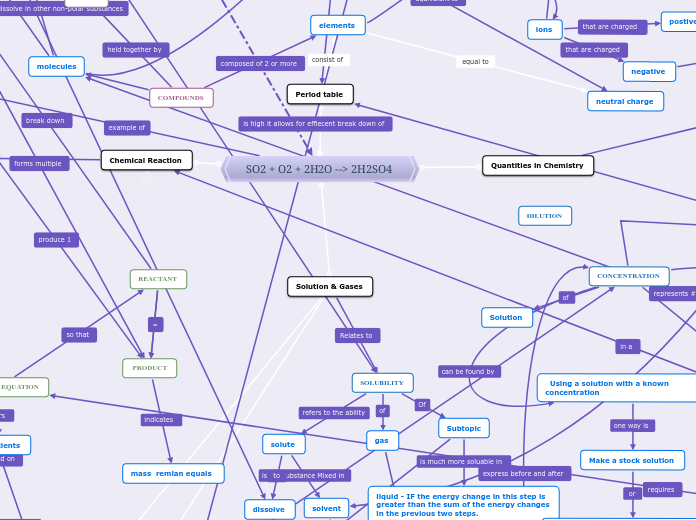
CONCENTRATION
Solution
Using a solution with a known concentration
Make a stock solution
dilute by adding known amount of water
Dilution
r
Percentage by mass
Percent by volume
molar concentration
DILUTION
ATMOIC NOTATION
symbol
PERIODIC TRENDS
KINETIC MOLECULAR THEORY
ACIDS AND BASES
PH scale
acids
Hydogen ions
base
protons
OH ions
water and salt
MOLE
Stoichiometry
Final product
percent yield
mol to mol
Coefficient
mole ratio
Reactants
Excess reactant
Limiting reactant
product
Yield
percen yield
Actual yield
theorotical yield
Product
Substances
SOLUBILITY
liquid - IF the energy change in this step is greater than the sum of the energy changes in the previous two steps.
gas
soluable
solute
solvent
solution
Saturated
the maximum amount of solute that can be dissolved in a solvent at a given temperature and pressure
unsaturated
solution in which more solute can be dissolved in the solvent at a given temperature and pressure
supersaturated
more than the maximum amount of solute that can be dissolved in a solvent at a given temperature and pressure
dissolve
Temperture
kinetic energy
PRODUCT
mass remian equals
REACTANT
BALANCING EQUATION
coefficients
6 TYPES OF REACTION
Single displacement
a more active element replaces the least active element
Subtopic
Neutraliation
double displacement
solubility chart
water
Precipitate
Combustion
Hydrcarbon
Oxygen
Carbon dioxide
Water
Decompostion
synthesis
POLARITY
molecular polarity
Bond polarity
BONDS
ionic bonds
metals and non metals
covalent bonds
COMPOUNDS
molecules
polyatomic
diatomic
ions
share valence electrons
Number of moles can be found by you use m/M. Molar mass is determined using m/n
Summary
SO2 + O2 + 2H2O --> 2H2SO4
Period table
elements
neutral charge
Quantities in Chemistry
Solution & Gases
gas laws
Tiration
Chemical Reaction
No mass is lost or gained
Law of conservation
Chemical equation
word equation