4.2 Standards
To name your story, you have to think about the overall message and what you want your audience to understand from the story. Also, make it relevant and easy to remember.
Dilution
How to do?
How many cm3 of a 10.00M HCl stock solution would you use to prepare 5dm3 of 0.5M HCl
M1 = 10.00 M M2 = 0.5 M
V1 = ? V2 = 5 dm3
M1V1 = M2V2
(10)V1 = (0.5)(5)
V1 = 0.25 dm3
Formula
M1V1 = M2V2
A procedure for preparing a less concentrated solution from a more concentrated one by adding a solvent
Standardization
Process
1. Weight out 1.325g of Na2CO3
2. Dissolve it in water
3. Add enough water to fill 250mL volumetric flask
4. Use this solution to standardized the HCl acid
A process to determine the concentration of a solution by titrating with a primary standard or with a solution of known concentration
Secondary Standard
The ending of a story is essential. We all know that if the ending is weak, what happened before loses its importance. So make it unpredictable, but fair. A resolved ending answers all the questions and ties up any loose threads from the plot.
Sodium hydroxide (NaOH)
This is the closure section of the story.
See examples of possible outcomes below:
- all problems have been solved
- it's clear how each one of your characters ends up
- your main character is transformed by the challenge
Usually cheap and easy to use
Usually powerful reactants
Try answering these questions in order for you to come up with a closure:
- Have all problems been solved?
- Is it clear what happens with all your characters in the story?
- Has the challenged transformed your main character?
- How do the characters feel in the end?
Concentration change over time
Secondary standard are influenced atmosphere / environment
Try answering these questions to come up with a closure:
- Have all the problems been solved?
- Is there a clear picture of what happens with each character in the story?
- Has the challenge transformed your main character?
- How do the characters feel in the end?
This is the moment when the main character surpasses the last obstacle and finally faces their greatest challenge.
The climax usually follows one of these patterns:
- realization
- resolution
- choice
Type in your answer.
A reagent (usually prepared in the laboratory) whose purity has been established by standardizing it against a primary standard
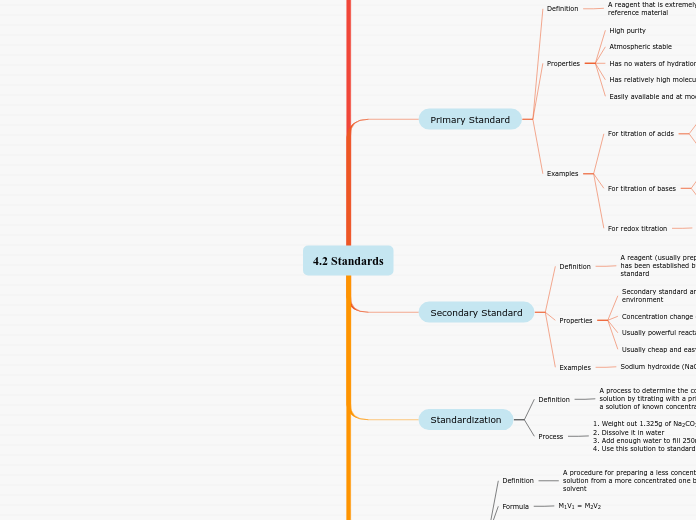
Primary Standard
The middle of the story is where you add layers of complications that will lead to the end. Reveal more about the character's journey. Did their personality go through changes? How did they overcome the challenges? And as you build up the story’s central conflict, make it more personal to that character. Also, from the middle act, you have to lead into the final act.
Examples
There wouldn't be any tension and excitement in your story if there weren't any obstacles in your character's way.
For redox titration
potassium dichromate : K2Cr2O7,
mol wt= 294.19 g/mol
For titration of bases
ii) potassium hydrogen iodate : KH(IO3)2,
mol wt= 289.92 g/mol
i) potassium hydrogen phthalate (KHP): KHC8H4O4, mol wt= 204.23 g/mol
For titration of acids
A story is nothing more than a character overcoming a series of difficulties to reach the desired goal. Obstacles usually create suspense and conflict. In overcoming obstacles, there is growth: weak becomes strong; hatred turns into love; sadness into happiness; wrong into right; lies into truth; or evil becomes good.
See a few examples below:
- stopping a meteor
- finding a killer
- finding love
ii) tris-(hydroxymethyl)aminimethane,
(CH2OH)3CNH2 mol wt= 121.12 g/mol
i) Sodium carbonate, Na2CO3,
mol wt= 105.99 g/mol
Properties
Your character(s) need(s) motivation in order to solve the challenge(s).
Easily available and at modest cost
Has relatively high molecular weight
Has no waters of hydration (non hydroscobic)
Atmospheric stable
High purity
Secondary characters also might have motivs beacuse of which they may cross path with main character or which might trigger them to help the main character.
Each story has a main character and that character usually needs to solve a problem or challenge. The character's challenge is the one that creates tension throughout the story.
A reagent that is extremely pure and serve as a reference material
In most stories, there are 3 challenges. The number 3 is a mystical number symbolizing completeness. Try to come up with interesting challenges with which your character needs to struggle.
See a few examples below:
- turns into a werewolf at night
- is sent back in time
Definition
In the beginning of the story (or the exposition), you will need to introduce the setting and characters. You might also want to introduce the main conflict. This part of the story is important because it gives the reader necessary background information and maybe even a first insight into a character’s personality.
Working Solution
A name given to a chemical solution made for actual use in the lab, usually made from diluting or combining stock or standard solution
Stock Solution
The setting (time & place) of a story can change throughout the plot.
A concentrated solution that will be diluted to some lower concentrated for actual use
Sensory details include sight, sound, touch, smell, and taste. These details are important because they create depth in your setting.
See a few examples below:
- the smell of fresh bread
- the scent of freshly cut grass
- rain falling onto the windshield etc.
Standard Solution
Characters are essential to a good story. Usually, the protagonist(s) is/are the most affected by the plot. Introduce a character by focusing on their actions, interests, and occupation, as the physical appearance doesn't make a difference in most cases.
A reagent solution of accurately known concentration
Type in the name of your character.