Da
1/12 mass
Carbon 12 atom
Bohr Rutherford
spherical
Water
Hydrogen gas + Base
Acid
Heat+Light
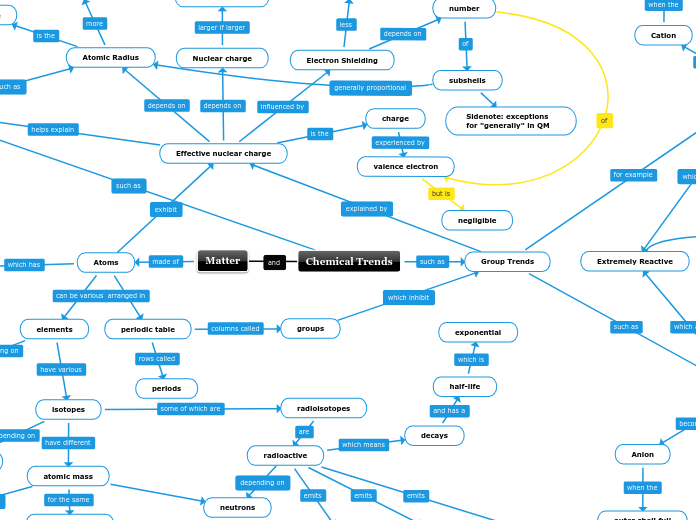
Effective nuclear charge
Trends
Ionisation Energy
atomic number
increases
energy
an electron
Electronegativity
tendency to attract
bond
group
Electron Affinity
electron is attached
period
charge
valence electron
negligible
Atomic Radius
distance
down the group
across the period
small
picometer
closer
Electron Shielding
number
Sidenote: exceptions for "generally" in QM
ENC
more electrons
Nuclear charge
Atomic Number
Protons
electrons
Coulomb's Law
Matter
Atoms
periodic table
periods
groups
elements
isotopes
atomic mass
same element
neutrons
radioisotopes
radioactive
gamma radiation
beta radiation
alpha radiation
decays
half-life
exponential
neutron count
proton count
Atomic Structure
Quantum Model
Electron
clouds of uncertainty
shells
subshells
cloverleaf
dumbbell
sphere
heisenberg uncertainty principle
momentum
position
same time
-1
Neutron
no charge
nucleus
centered in atom
Proton
+1
elementary charge
smallest possible charge
positively charged
Simplified models
diagrams
Conceptualization
properties, trends, etc
Chemical Trends
Group Trends
Group 17
Anion
1 Electron
Stable shell
Extremely Reactive
Halogens
Non metals
Group 1
Cation
outer shell full
More reactive
Alkali Metals
Metals