Another function that was not added until later is cell signaling, with examples like the kinase receptors
The cell membrane is composed of many lipids, some with glycerol (a three carbon molecule) acting as the backbone
Gene regulation occurs in the nucleus of the cell because this is where transcription occurs
Cellular Respiration
Oxidative Phosphorylation
Chemiosmosis ATP Synthesis
concentration gradient
transport protein - ATP Synthesis
Electron Transport Chain
Inner mitochondrial membrane
Anerobic respiration
Lactic Acid
Alcohol Fermentation
Substrate level phosphorylation
Pyruvate Oxidation
c
Citric acid cycle begins
Citrate
Isocitrate
a-ketoglutarate
Succinyl CoA
Succinate
Fulmarate
Malate
Oxaloacetate
Glycolysis
Energy Payoff Phase
NAD+
Pyruvate
Water/H2O
NADH
Protons
Phosphate
Electrons
Energy Investment Phase
ADP
ATP
Replication forks- located at each end
of the replication bubble
Topoisomerase- enzyme
DNA Replication
Double stranded DNA- with a complimentary, antiparallel structure
Helicase- enzyme
Single-stranded DNA
Single-stranded Binding
Protein (SSBs)- enzyme
Primase-enzyme
Lagging strand
Okazaki fragments
DNA ligase- fills in the gaps left by
removed RNA primase with nucleotides
complimentary to the parent strand
Leading strand
Parental DNA
DNA polymerase III
Daughter strand
5' to 3' direction
Chargaff's rule- states purines and pyrimidine must be paired with one another, specifically the same proportion of
adenine to thymine and guanine to cytosine
sliding clamp
Origin of replication: Sequence of nucleotides that indicates
the beginning of DNA replication
Replication bubble
Bidirectional forks- located at each
end of the replication bubble
Fast and accurate
Concept Map 3
Transcription
Termination
the pre-mRNA is noticed by ribonuclease an amino acid which cuts the pre-mRNA from the DNA
a 5'cap is added to the pre-mRNA
a 3' poly-A tail is added
by polyA polymerase
the pre-mRNA is now ready
to undergo processing
RNA Processing
introns are cut out by spliceosome
exons are brought together through spliceosome
mature mRNA is created
and ready for translation
into proteins
exon-material containing suitable nucleotides for protein function
intron-filler material
Elongation
RNA polymerase ii bind to the template strand of DNA 3'-5'
RNA polymerase ii unwinds DNA and then adds RNA nucleotides
the RNA being created resembles the non-template strand of DNA 5'-3'
pre-mRNA strand is now created 5'-3'
Initiation
Promoter
start point-point of transcription
TATA box-using for recognition of transcription factors
Transcription Factors-used to RNA polymerase ii can bind in the correct position
RNA Polymerase ii-add RNA nucleotides
RNA polymerase ii and Additional transcription factors bind in order to make a transcription initiation complex.
Concept Map 2
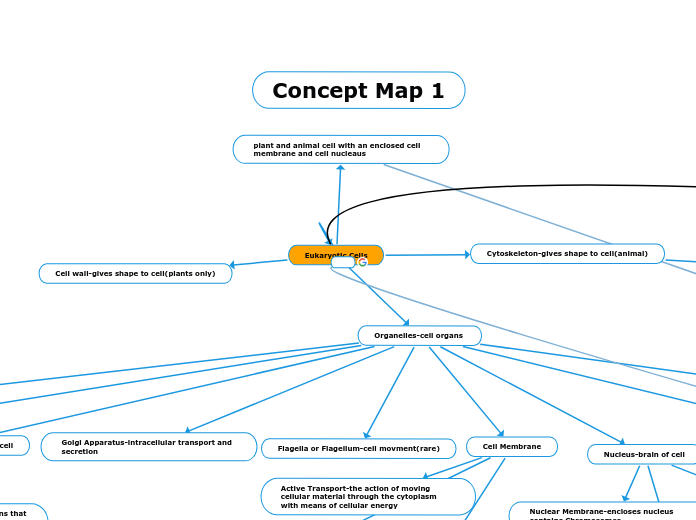
Concept Map 1
Prokaryotes
Prokaryotes - A type of organism that is single celled, lacks a nucleus, and contains no membrane bound organelles.
Metabolism
Facultative anaerobes
This kind of organism utilizes aerobic respiration when oxygen is present, but can also use fermentation when oxygen is not.
Obligate anaerobes
Organisms that cannot tolerate environments with oxygen present as it would lead to cell death and instead utilize fermentation anaerobic respiration.
Obligate aerobes
Organisms that require oxygen for cell respiration
Common Structures
Nucleoid
Prokaryotic cells don't have a membrane bound organelle for their DNA, rather they have a recognized region where DNA is located known as the nucleoid.
Materials Present
Controls the synthesis of proteins and provides essential messenger functions for DNA.
Its structure varies from DNA in that it has ribose sugars instead of deoxyribose, is single-stranded, and has uracil instead of thymine
Bacterial Genome
Endospore
Endospores are essential for bacteria living under extreme conditions. It protects the DNA of the cell by making a dormant and resistant cell that can survive various circumstances.
As the rest of the cell disintegrates, the DNA is protected by the multiple layers of cell wall material surrounding it, forming the endospore.
Gas vacuoles
Gas vacuoles assist in buoyancy for flotation in aquatic environments.
Capsules and slime layers
This structure surrounds the cell wall of bacteria as a sticky later of polysaccharide or protein, and helps the cell for adhesion and protection.
Flagella
The flagella is important for the cell's motility, and contains a hook a basal body. It can exist either around the surface of the cell, or be concentrated in one area.
Ribsomes
Ribosomes are present in both prokaryotes and eukaryotes, and are essential for protein synthesis.
Cytoplasm
The cytoplasm consists of gel-like cytosol, water based solution with ions and small molecules, and makes up the internal part of the cell inside the plasma membrane.
Cell wall
Present in all prokaryotes, the cell wall maintains the cell's shape, and provides protection and support. This structure is made of modified sugars with short polypeptides called peptidoglycan.
Peptidoglycan
This substance that makes up the cell wall is unique to bacteria, and can be used to target them specifically.
Fimbriae and pili
Fimbriae are short abundant structures used by bacteria to stick to other bacteria, while longer pili assist in transferring DNA between cells.
Plasma membrane
The plasma membrane is a selectively permeable barrier for the cell. It provides boundaries between the inside of the cell and its environment, facilitates the transport of nutrients and waste, and is where metabolic processes occur.
Factors of fluidity
The weak Van der Waal forces present in the membrane allow for the movement of lipids. The fluidity of the membrane however is determined by a few factors.
Cholesterol embedded within the plasma membrane allows for the regulation of the movement in phospholipids by reducing movement at moderate temperatures and preventing the overpacking of saturated fatty acids at low temperatures by acting as a buffer.
Types of fatty acids present
The ratio of unsaturated fatty acids to saturated fatty acids also affects the fluidity of the membrane. Saturated fatty acids lack the structural kink that is present in unsaturated fats, allowing the phospholipids to pack together more tightly, and resulting in a more rigid structure.
Temperature
At a specific lower temperature, the membrane's phospholipids change from their more liquid, crystalline phase, to a more gel-like phase.
Transport of molecules
The membrane being selectively permeable means only certain molecules can pass through without assistance.
Molecules that can transport across the membrane
- Small non polar
- Small uncharged
Molecules that cannot
- Large uncharged polar molecules
- Ions
Specific structures
The plasma membrane is made up of an amphipathic phospholipid bilayer, with hydrophilic heads bordering the outside and inside of the cell, and the hydrophobic tails making up the interior of the membrane.
This arrangement is held together by weak Van der Waal forces that allow lipids to move within the membrane and provides fluidity.
The membrane also consists of proteins located on the surface (surface proteins), within the membrane (integral proteins), and with chains of polypeptides (glycoproteins).
Types
Archaea
A category of prokaryotic micro-organisms
Methanogens
Another prokaryote in the archaea domain are methanogens. These can be found in hydrothermal vents, the guts of animals, and wetlands. They are anaerobic, meaning oxygen is poisonous to them, and remove excess hydrogen.
Extreme thermophiles
This kind of prokaryote can thrive in high temperatures that few other organisms can survive in. They are commonly found in many extreme temperature environments such as hot springs and hydrothermal vents.
Extreme halophiles
These kinds of archaea are able to thrive in extremely saline environments, referred to as extreme as very little amounts of organisms can survive in similar conditions.
Bacteria
Nutrition Modes
Heterotrophs
Chemoheterotroph
Chemoheterotrophs utilize carbon from organic compounds, and are characteristics of many prokaryotes.
Photoheterotroph
The prefix photo refers to light, which this mode utilizes for energy. This trait is unique to certain aquatic and salt thriving prokaryotes. Since its a heterotroph it utilizes carbon from organic compounds.
Autotrophs
Chemoautoroph
Inorganic chemicals
This mode, as an autotroph, means a prokaryote gets its energy from inorganic chemicals.
Photoautorophs
Light
Organisms that utilize light to synthesize organic molecules are known as photoautotrophs, such as cyanobacteria.
Cell membranes
Structures
made of
proteins
transport, enzymatic activity, cell-cell recognition, signal transduction, intercellular joining, and attachment to the cytoskeleton and extracellular matrix (ECM)
Transmembrane proteins have an extracellular and cytoplasmic side
Ion channels
sodium potassium-pump, potassium channel, sodium channel
Gated (stretch-gated, ligand-gated, voltage-gated)
Un-gated (always open)
phospholipid bilayer
hydrophobic tail (fatty acid chain) and hydrophilic head (polar) which help control membrane fluidity
are
selectively permeable
Function
barrier, support, protection
separates organism from the environment
transport
larger molecules
exocytosis
endocytosis
receptor-mediated
phagocytosis
pinocytosis
smaller molecules
osmosis/diffusion
Hypertonic solution
plasmolyzed (plant loses water)
Hypotonic solution
turgid(normal in a plant cell)
Isotonic
active transport
ex:sodium-potassium pump
low->high concentration
protein pumps
facilitated diffusion
channel proteins are needed
passive transport facilitated by proteins
Floating topic
Biomolecules
Molecules created by
living organisms.
hydrolysis
dehydration reaction
Proteins
Polypeptide
Quaternary: 2 or more
polypeptides form a
functional protein through
R group interactions
Tetramer: 4 PPs
Trimer: 3 PPs
Dimer: 2 PPs
Monomer: 1 PP
Tertiary: R groups interact
to from 3D shape
Disulfide bond
Ionic bonds
Hydrophobic
interactions
Hydrogen bonds
Secondary: Main chains form
hydrogen bonds
Beta-pleated sheet
Alpha helix
Primary: Amino acids
connected through peptide
bonds
Amino acids
Side chain:
R group
Basic: Complete
positive charge
Acidic: Complete
negative charge
Nonpolar: Has H, CH,
or carbon ring
Polar: Has OH, SH,
or NH groups
Main chain: Amino &
carboxyl groups
Lipids
Phospholipids
Form bilayer in water
Hydrophilic head:
Phosphate group
Hydrophobic tail:
Glycerol &
2 Fatty acids
Steroids
Testosterone
Cholesterol
LDL: Carries excess to
blood vessels (bad)
HDL: Carries excess to
liver for excretion (good)
4 fused rings
Triglycerides
Energy storage
3 Fatty acids
Unsaturated
Trans: H on different sides
of double-bonded carbons
Cis: H on same side
of double-bonded carbons.
Has a kink
Liquid at room temp
One or more
double bonds &
H atoms are notat every position
Saturated
Solid at room temp
No double bonds & H atoms
at every position
Glycerol
Nucleic Acids
Gene expression
RNA
nitrogenous
base
Cytosine (C) & Uracil (U)
ribose sugar
DNA
nitrogenous base
Pyrimidines
Cytosine (C) & Thymine (T)
Purines
Adenine (A) & Guanine (G)
phosphate
deoxyribose sugar
Carbohydrates
Complex
Structure polysaccharides
Chitin
Cellulose
Beta glucose & 1-4 glycosidic
linkages (no branching)
Storage polysaccharides
Dextran
Starch
Amylase
Alpha glucose & 1-4 and 1-6
glycosidic linkages (some branching)
Amylose
Alpha glucose & 1-4 glycosidic
linkages (no branching)
Glycogen
Alpha glucose & 1-4 and 1-6
glycosidic linkages (lots of branching)
Simple (Sugars)
Disaccharides
Maltose
Lactose
Sucrose
Monosaccharides
Hexoses: 6-carbon
Fructose
Galactose
Glucose
Beta: OH is above the ring
Alpha: OH is below the
ring
Pentoses: 5-carbon
Deoxyribose
Ribose