por Yun Shan 13 anos atrás
1581
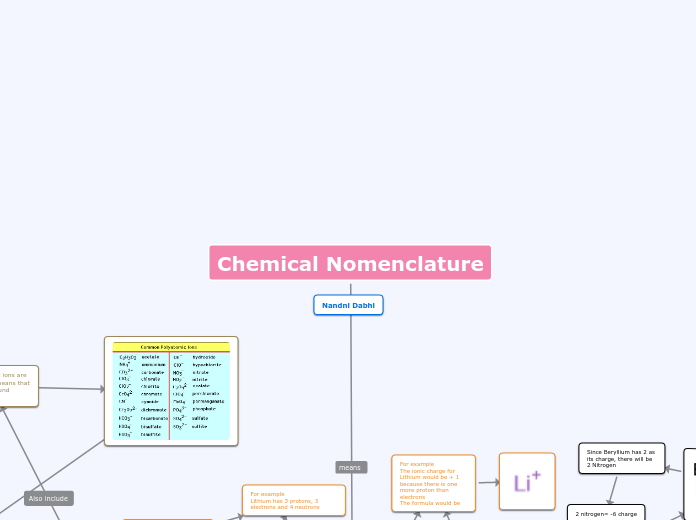
Chemcial/ionic bonding
A chemical bond represents the attraction between atoms, primarily influenced by the behaviors of their outermost electrons. These behaviors result in various types of bonds, with ionic and covalent bonds being the most significant.