por Anika Bradley 5 anos atrás
377
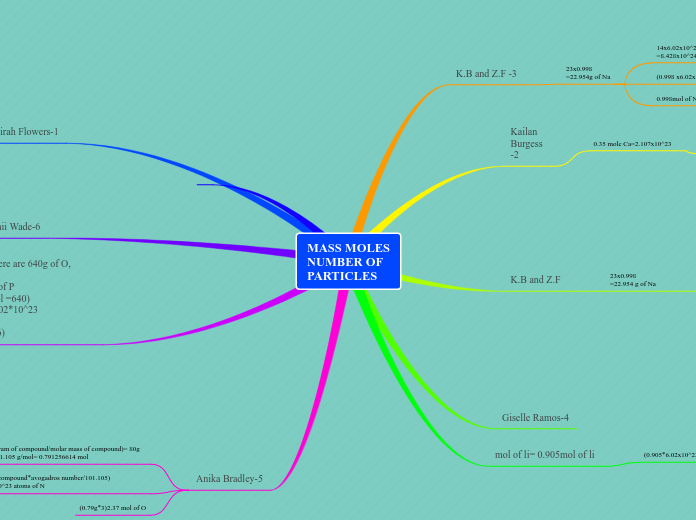
Mass Moles Number of Particles Assignment
The text provides a series of calculations involving different chemical elements and compounds, focusing on their masses, moles, and the number of particles. It includes various examples, such as sodium (