по Siti Zahrah 6 лет назад
2109
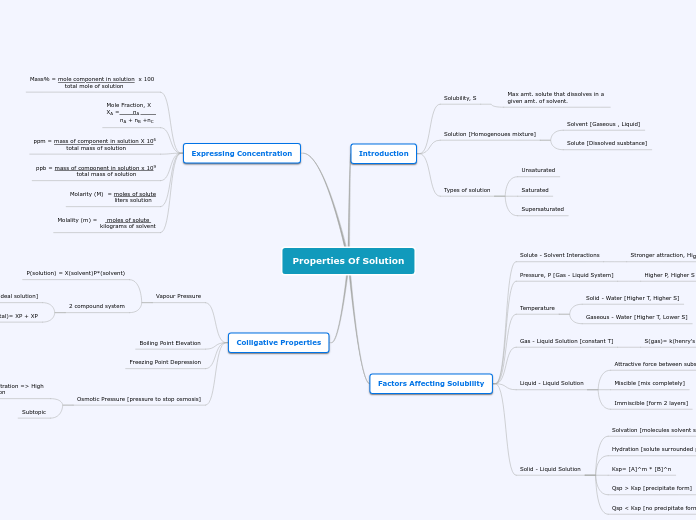
Properties Of Solution
Solutions are homogeneous mixtures consisting of solutes dissolved in solvents. The concentration of these solutions can be measured in various ways, including molality, molarity, and parts per million (