作者:Sebastian Birch Schmidt 12 月以前
103
Halides
The discussion revolves around the properties and behaviors of various minerals and their interactions with cations, particularly focusing on the differences in ion sizes such as Al+3 and Fe+.
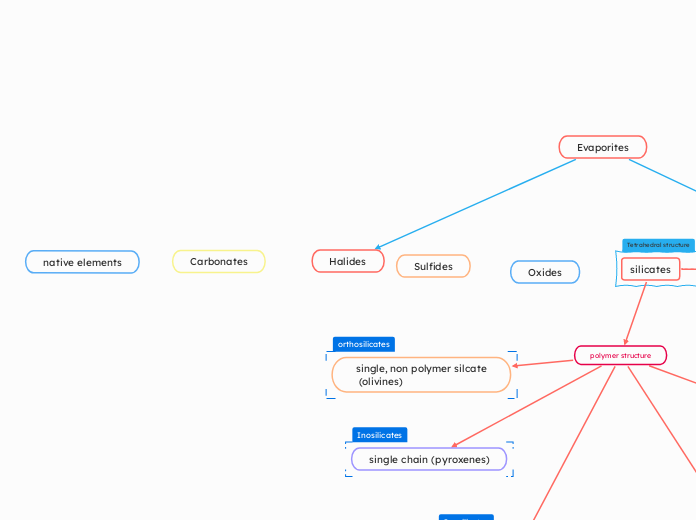
作者:Sebastian Birch Schmidt 12 月以前
103

更多类似内容