door Sebastian Birch Schmidt 12 maanden geleden
102
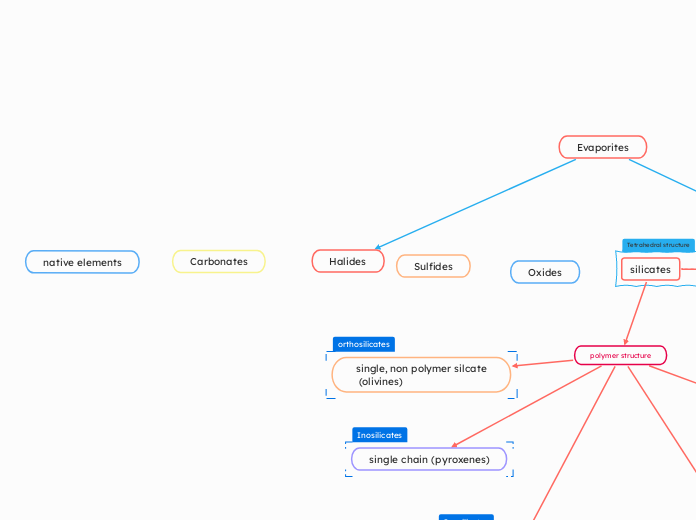
Halides
The discussion revolves around the properties and behaviors of various minerals and their interactions with cations, particularly focusing on the differences in ion sizes such as Al+3 and Fe+.